Key Points
MRZ is an irreversible pan-proteasome inhibitor that has antitumor activity.
Two treatment regimens were explored in a phase 1 trial in patients with RRMM.
Abstract
Marizomib (MRZ) is a novel, irreversible proteasome inhibitor in clinical development for the treatment of relapsed or relapsed and refractory multiple myeloma (RRMM). MRZ inhibits the 3 proteolytic activities of the 20S proteasome with specificity distinct from bortezomib and carfilzomib. Study NPI-0052-101 Part 1 enrolled relapsed or RRMM patients into an open-label, dose-escalation design to determine the maximum tolerated dose and recommended phase 2 dose (RP2D) of MRZ administered intravenously on 2 different schedules: schedule A (0.025-0.7 mg/m2 once weekly on days 1, 8, and 15 of 4-week cycles) and schedule B (0.15-0.6 mg/m2 twice weekly on days 1, 4, 8, and 11 of 3-week cycles; concomitant dexamethasone was allowed with schedule B). Patients had received an average of 4.9 and 7.3 prior treatment regimens (schedules A and B, respectively). MRZ schedule A was administered to 32 patients, and the RP2D was established as 0.7 mg/m2 infused over 10 minutes. Schedule B was administered to 36 patients, and the RP2D was determined to be 0.5 mg/m2 infused over 2 hours. The most common (>20% of patients) related adverse events were fatigue, headache, nausea, diarrhea, dizziness, and vomiting. Six patients achieved clinical benefit responses (defined as minimal response or better), including 5 partial responses (1 patient on schedule A and 4 on schedule B; 3 of these 4 patients received concomitant dexamethasone). MRZ was generally well tolerated, and results suggest activity in previously treated RRMM patients. Combination studies using pomalidomide and dexamethasone are now underway. The trial was registered at www.clinicaltrials.gov as #NCT00461045.
Introduction
The proteasome is a multicatalytic proteinase complex responsible for degradation of a wide variety of protein substrates within normal and transformed cells. It is a well-validated target in multiple myeloma (MM). Three proteasome inhibitors are currently approved for treatment of MM (bortezomib [BZ], carfilzomib [CFZ], and ixazomib),1-8 and others are currently in development. Although BZ-, CFZ-, and ixazomib-based therapies are important advances, they are associated with toxicities9-14 and the development of acquired resistance.4-8,15-17 In addition, almost all MM patients who have received several lines of therapy eventually relapse and exhibit progressively lower rates and durations of response as increased resistance emerges.18 Therefore, current therapeutic options for patients with relapsed and refractory disease are limited, and effective treatments that reestablish tumor responsiveness are urgently needed.19,20
Marizomib (MRZ; also known as NPI-0052 or salinosporamide A) is a broad-spectrum proteasome inhibitor that displays antitumor activity in vitro and in vivo. MRZ inhibits the 3 separate enzyme activities of isolated proteasomes, chymotrypsin-like (CT-L), trypsin-like (T-L), and caspase-like (C-L), with half-maximal inhibitory concentration values of 3.5, 28, and 430 nM, respectively.21 MRZ also blocks all 3 proteasome subunits (CT-L, T-L, and C-L) in MM and in other cancer cell lines, where its activity has been shown to be distinct from that of BZ.21 MRZ shows activity in MM cells that are resistant to a variety of clinical drugs, including BZ, thalidomide, and dexamethasone (Dex).21 In vivo, MRZ inhibits proteasome activity in whole blood, solid tissues, and human MM tumors when administered IV or orally to nude mice and exerts antitumor activity in various MM models both alone21,22 or in combination with BZ, lenalidomide, or pomalidomide.22-24 MRZ penetrates the blood–brain barrier and inhibits proteasome activity in the central nervous system (CNS) with minimal cytotoxic effects on normal human neural stem and progenitor cells compared with malignant glioma stem-like cells or established glioma cell lines.23
On-target mechanisms of acquired resistance to proteasome inhibitors that have been described include resynthesis and upregulation of the dominant CT-L subunit25 and compensatory increases in activity of the C-L and T-L subunits.26 Due to its pan-subunit inhibitory activity in MM cells and irreversible binding,27-29 MRZ has the potential to overcome both of these mechanisms clinically and to improve patient outcome. This study presents the results of a phase 1 clinical trial of MRZ in patients with relapsed or relapsed and refractory multiple myeloma (RRMM).
Patients and methods
Study design
Study NPI-0052-101 was divided into 2 parts. Part 1 was a phase 1, multicenter, open-label study of MRZ in patients with relapsed MM or RRMM. Part 2 (to be reported separately) was a phase 2 study in patients who received and were refractory to prior CFZ.
The primary objective of this study was to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of MRZ when administered IV to patients with relapsed or RRMM. The MTD was defined as the dose level below that which caused ≥2 protocol-defined dose-limiting toxicities (DLTs) in ≤ 6 patients. The MTD or a lower dose could be selected as the RP2D depending on safety, pharmacokinetic (PK), and pharmacodynamic data. After determination of the MTD, additional patients were to be treated at the RP2D to further define safety and toxicity. Anti-tumor activity was also assessed in this study, using the modified European Group for Bone Marrow Transplantation (EBMT30 ) and the International Myeloma Working Group (IMWG) Uniform Response Criteria.31-33 Evaluations of the PK of repeated dosing and biological activity of MRZ, including serial assessment of proteasome inhibition were conducted and will be reported separately.
Study NPI-0052-101 Part 1 comprised 5 stages: stage 1 was a 3+3 dose escalation to determine the schedule A (once-weekly) MTD or RP2D, stage 2 comprised expansion of the RP2D up to 12 patients, stage 3 introduced a lyophile formulation; stage 4 was schedule B (twice-weekly) dose escalation, and stage 5 was expansion of up to 24 patients treated at the schedule B RP2D.
Patient eligibility
This study enrolled relapsed or RRMM patients age ≥18 years and with Karnofsky performance status ≥70%. Patients could have undergone prior autologous bone marrow transplantation.
Dose-escalation cohorts enrolled patients with relapsed or RRMM disease for which no other approved treatment was available and clinically indicated. Relapsed or RRMM patients enrolled in RP2D cohorts were required to have received at least 2 prior treatment regimens, including at least 2 cycles of lenalidomide and at least 2 cycles of BZ (either separate or within the same regimen), and also received a cytotoxic chemotherapy agent (eg, alkylating agent). Relapsed MM patients were those who had progressive disease (PD) after having achieved at least stable disease for at least 1 cycle during 1 prior regimen. Refractory MM patients were those with documented PD within 60 days of completing the most recent regimen.
Measurable disease was not required for dose-escalation patients (schedule A or B). Measurable relapsed or relapsed and refractory disease34 was required for patients enrolled at the RP2D for both schedules. Measurable disease was defined as serum M-protein ≥0.5 g/dL or urine M-protein ≥200 mg/24 hours or involved serum free light chain level ≥10 mg/dL, provided the serum free light chain ratio was abnormal).
Laboratory eligibility criteria were consistent with adequate hematologic, renal, and liver function and were modified by protocol amendment as information was learned from this and separate MRZ trials (NPI-0052-100 [NCT00396864], patients with solid tumors or refractory lymphoma; and NPI-0052-102 [NCT00629473], patients with solid tumors or advanced hematologic malignancies).
Eligibility criteria required washout times prior to start of study treatment of chemotherapy, biological immunotherapy, radiation therapy, transplantation, and investigational agents to account for possible ongoing activity or toxicity of these agents.
Early eligibility criteria excluded concomitant oral prednisone >10 mg/day or intrathecal therapy, ongoing coagulopathies and/or anticoagulants, evidence of mucosal or internal bleeding and/or platelet-refractory documented adrenocortical insufficiency or preexisting pancreatitis, untreated urinary tract infection, abnormal urine sediment, and preexisting kidney disease (parameters were imposed to exclude patients with substantial renal impairment including creatinine and creatinine clearance limits and protein level evaluations to detect urinary casts suggestive of glomerular nephritis). As more safety information was gained, protocol amendments allowed anticoagulants for treatment of prior deep vein thrombosis, mineralocorticocoid replacement therapy, bisphosphonates, and low-dose corticosteroids.
Eligibility criteria also excluded significant cardiac disease, prior hypersensitivity reaction to therapy containing propylene glycol or ethanol; bacterial or fungal infection requiring systemic therapy; infection requiring parenteral antibiotics; known positivity for HIV, hepatitis A, B, or C; and any medical conditions that would impose excessive risk to the patient. Pregnant or breast-feeding women were excluded, as were patients unwilling or unable to comply with protocol procedures.
This study was conducted according to the Declaration of Helsinki and was approved by the centers’ relevant institutional review boards or ethics committees. All participants gave written informed consent. The www.clinicaltrials.gov registered title is “Phase 1/2 Clinical Trial of NPI-0052 in Patients with Relapsed or Relapsed/Refractory Multiple Myeloma” (#NCT00461045).
Treatment
Dose levels for this study were modified (eg, expanded or concluded early) as information was learned from this and separate MRZ trials (NPI-0052-100 and NPI-0052-102). Table 1 outlines the dosing schedules investigated. The schedule A regimen (MRZ administration over 1 or 10 minutes on days 1, 8, and 15 in 4-week cycles) used in stages 1-3 was the initial selected regimen based on prolonged proteasome inhibition by MRZ and therapeutic similarity of weekly compared with twice-weekly administration in animal models. Schedule A infusion time was increased from 1 to 10 minutes with amendment 4 because, when administered over 1 minute, larger infusate volumes intermittently elicited transient peri-infusional pain due to viscosity of solution. The second dosing regimen, schedule B (MRZ administration over 1 or 2 hours on days 1, 4, 8, and 11 in 3-week cycles), used in stages 4 and 5, was added with amendment 6 to evaluate whether more frequent dosing would be optimal. Infusion time was increased from 10 minutes to 1 hour with amendment 6 because infusion discomfort was occasionally observed over 10 minutes and was further increased to 2 hours with amendment 7 to empirically assess the ability of longer infusion times to ameliorate toxicity and increase MTD. To mitigate renal dysfunction, patients received normal saline prior to and following MRZ administration. Schedule B patients who did not achieve at least a minimal response (MR) after cycle 2 were allowed to receive concomitant Dex (20 mg) (amendment 10 changed Dex administration to after cycle 1, to slow PD). Dex was administered the day of and the day after MRZ.
Treatment regimens
| Stages and dosing schedule . | Regimen . | Formulation used . | Cohort . | Doses . | Number of patients who received Dex* . | Number of patients who received MRZ† . |
|---|---|---|---|---|---|---|
| Stages 1-3: schedule A, once weekly | Days 1, 8, and 11, 4-wk cycles, 1 or 10 min | IV liquid | −3 | 0.025 mg/m2 | 0 | 3 |
| −2 | 0.05 mg/m2 | 0 | 3 | |||
| −1 | 0.075 mg/m2 | 0 | 7 | |||
| 1 | 0.15 mg/m2 | 0 | 2 | |||
| 2 | 0.3 mg/m2 | 0 | 1 | |||
| 3 | 0.6 mg/m2 | 0 | 3 | |||
| 4/RP2D§ | 0.7 mg/m2 | 0 | 8 | |||
| IV lyophile‡ | Expanded RP2D*,§ | 0.7 mg/m2 | 0 | 5 | ||
| Stages 4 and 5: schedule B, twice weekly | Days 1, 4, 8, and 11, 3-wk cycles, 1 or 2 h | IV lyophile | 1 | 0.15 mg/m2 | 1 | 3 |
| 2 | 0.4 mg/m2 | 3 | 4 | |||
| 3 | 0.5 mg/m2 | 1 | 2 | |||
| 4 | 0.6 mg/m2 | 3 | 3 | |||
| RP2D§ | 0.5 mg/m2 | 15 | 24 |
| Stages and dosing schedule . | Regimen . | Formulation used . | Cohort . | Doses . | Number of patients who received Dex* . | Number of patients who received MRZ† . |
|---|---|---|---|---|---|---|
| Stages 1-3: schedule A, once weekly | Days 1, 8, and 11, 4-wk cycles, 1 or 10 min | IV liquid | −3 | 0.025 mg/m2 | 0 | 3 |
| −2 | 0.05 mg/m2 | 0 | 3 | |||
| −1 | 0.075 mg/m2 | 0 | 7 | |||
| 1 | 0.15 mg/m2 | 0 | 2 | |||
| 2 | 0.3 mg/m2 | 0 | 1 | |||
| 3 | 0.6 mg/m2 | 0 | 3 | |||
| 4/RP2D§ | 0.7 mg/m2 | 0 | 8 | |||
| IV lyophile‡ | Expanded RP2D*,§ | 0.7 mg/m2 | 0 | 5 | ||
| Stages 4 and 5: schedule B, twice weekly | Days 1, 4, 8, and 11, 3-wk cycles, 1 or 2 h | IV lyophile | 1 | 0.15 mg/m2 | 1 | 3 |
| 2 | 0.4 mg/m2 | 3 | 4 | |||
| 3 | 0.5 mg/m2 | 1 | 2 | |||
| 4 | 0.6 mg/m2 | 3 | 3 | |||
| RP2D§ | 0.5 mg/m2 | 15 | 24 |
Dex, if administered, was instructed to be administered on the day of and the day after MRZ dosing.
Data from study NPI-0052-102 provided safety information to allow some dose levels (0.15 mg/m2 and 0.3 mg/m2) to be bypassed with fewer than 3 patients enrolled.
On schedule A, all patients who were dosed prior to the RP2D cohort received the liquid formulation; the 5 schedule A patients in the RP2D cohort received the lyophile formulation by crossover (ie, alternate assignment of patients to receive their first dose using either the lyophile or liquid formulation).
The schedule A RP2D was established as 0.7 mg/m2 infused over 10 minutes, and the schedule B RP2D was 0.5 mg/m2 infused over 2 hours.
Dose escalation
Prior to dose escalation to the next cohort in both schedules, the sponsor’s medical monitor and participating investigators reviewed all available safety data.
Schedule A of this study was conducted in parallel with separate MRZ trials (NPI-0052-100 and NPI-0052-102) that studied the same dosing schedule, and dose levels in this study were modified by protocol amendment as safety information was learned. The parallel-run trials later escalated to study higher doses of 0.8 and 0.9 mg/m2 at schedule A and dictated that the high schedule A dose in this trial be the 0.7-mg/m2 dose.
Schedule B patients were enrolled using a classic 3+3 study design. Within a cohort, if 1 out of 3 patients experienced a DLT, the cohort was increased to 6 patients. If no more than 1 out of 6 patients experienced a DLT, then the next dose was evaluated. If ≥2 patients in a cohort of 3-6 patients experienced a DLT, then the MTD had been exceeded and further accrual was at the previous dose; if this dose resulted in 0 of 6 patients without DLT, an interim dose could be attempted. The MTD or a lower dose could be selected as an RP2D depending upon safety, PK, and pharmacodynamic data.
The starting doses for schedules A and B were originally 0.1 mg/m2 and 0.15 mg/m2, respectively. The schedule A starting dose was based on animal safety studies and was decreased to 0.025 mg/m2 before patients were treated, because the first patient treated with MRZ in another phase 1 trial (NCT00396864) developed a serious adverse event (AE) of acute renal failure at 0.1 mg/m2 (the patient had multiple potentially contributive etiologies and preexisting renal dysfunction, and the association of MRZ to the serious AE was difficult to quantify). The schedule B starting dose was based on safety assessments of schedule A.
A patient could be treated for as long as he/she exhibited clinical benefit (stable disease or a tumor response) in the absence of unacceptable toxicity.
Statistical and analytical methods
The sample size of 35-70 patients was chosen based on recruitment considerations and methods of determining DLTs and MTD. It was estimated that 3 to 6 evaluable patients per dose level would provide data to assess the MRZ toxicity and PK profile, followed by up to 24 patients at the RP2D to confirm the effects of the drug prior to initiating phase 2.
DLT definition
DLT was defined as the occurrence of the following drug-related toxicities observed during cycle 1 (severity grades were assigned according to National Cancer Institute Common Terminology Criteria for Adverse Events v3.0): grade 4 neutropenia, anemia, or thrombocytopenia, despite transfusional or growth factor support of duration >5 days or febrile neutropenia; ≥2-grade shift from baseline in either pancreatic or neurotoxicity; grade 3 nausea, diarrhea, or vomiting in spite of maximal supportive care; clinically significant grade 3 or higher nonhematologic toxicity; or treatment delay ≥2 weeks due to prolonged toxicity. Patients with clinically significant grade ≥2 toxicity were required to delay treatment until toxicity recovered to entry criteria. Dose reductions were allowed for recurrent or specific toxicities.
Safety and efficacy analysis
Formal, comparative statistical analyses were not performed. All patients who received ≥1 MRZ dose were included in safety and efficacy (tumor response) summaries. All AEs and abnormal laboratory values were assessed according to Common Terminology Criteria for Adverse Events grading. For the final analyses of the safety of MRZ, all treatment-emergent AEs were summarized by system organ class and by preferred term using the Medical Dictionary for Regulatory Activities (MedDRA, v8.1). Each AE was reported by greatest severity and by strongest relationship to MRZ. Safety laboratory data included chemistries, hematology, and urinalysis.
Results
Demographics and baseline characteristics
Sixty-eight patients (32 schedule A and 36 schedule B), ages 38 to 82, were treated at 6 US sites between April 2007 and September 2013. Table 2 displays the demographic characteristics by schedule.
Summary of patient demographics for schedules A and B
| Characteristic . | Schedule A* (N = 32) . | Schedule B† (N = 36) . |
|---|---|---|
| Age (y) | ||
| Mean (SD) | 61.2 (9.62) | 62.3 (13.25) |
| Median | 61.0 | 61.0 |
| Minimum, maximum | 45, 82 | 38, 79 |
| Sex | ||
| Male | 20 (63%) | 22 (61%) |
| Female | 12 (38%) | 14 (39%) |
| Ethnicity | ||
| Hispanic or Latino | 0 | 1 (3%) |
| Not Hispanic or Latino | 31 (97%) | 34 (94%) |
| Unknown | 1 (3%) | 1 (3%) |
| Race | ||
| White | 27 (84%) | 29 (80%) |
| Black | 5 (16%) | 6 (17%) |
| Other | 0 | 1 (3%) |
| Characteristic . | Schedule A* (N = 32) . | Schedule B† (N = 36) . |
|---|---|---|
| Age (y) | ||
| Mean (SD) | 61.2 (9.62) | 62.3 (13.25) |
| Median | 61.0 | 61.0 |
| Minimum, maximum | 45, 82 | 38, 79 |
| Sex | ||
| Male | 20 (63%) | 22 (61%) |
| Female | 12 (38%) | 14 (39%) |
| Ethnicity | ||
| Hispanic or Latino | 0 | 1 (3%) |
| Not Hispanic or Latino | 31 (97%) | 34 (94%) |
| Unknown | 1 (3%) | 1 (3%) |
| Race | ||
| White | 27 (84%) | 29 (80%) |
| Black | 5 (16%) | 6 (17%) |
| Other | 0 | 1 (3%) |
SD, standard deviation.
Schedule A dosing was once weekly on days 1, 8, and 15 of 4-week cycles.
Schedule B dosing was twice weekly on days 1, 4, 8, and 11 of 3-week cycles; schedule B patients could receive low-dose Dex.
Varied oncology regimens were previously received by the relapsed or RRMM patients who were enrolled. The average numbers of prior oncology treatments were 4.9 and 7.3 for schedules A and B, respectively (medians: 4 and 6 [ranges 1-11 and 2-19], respectively). Prior BZ was received by 69% and 97% patients on schedules A and B, respectively (prior BZ was not required in dose escalation cohorts). Almost all patients had received a prior immunomodulatory drug (IMiD) (94% and 100%, schedules A and B, respectively), and over half of patients had ≥3 previous relapses (53% and 67% for schedules A and B, respectively).
Exposure and safety
MRZ schedule A doses included 0.025 (3 patients), 0.05 (3 patients), 0.075 (7 patients), 0.15 (2 patients), 0.3 (1 patient), 0.6 (3 patients), and 0.7 mg/m2/RP2D (13 patients); schedule B doses included 0.15 (3 patients), 0.4 (4 patients), 0.5 (2 patients), 0.6 mg/m2 (3 patients), and 0.5 mg/m2/RP2D (24 patients) (as presented in Table 1).
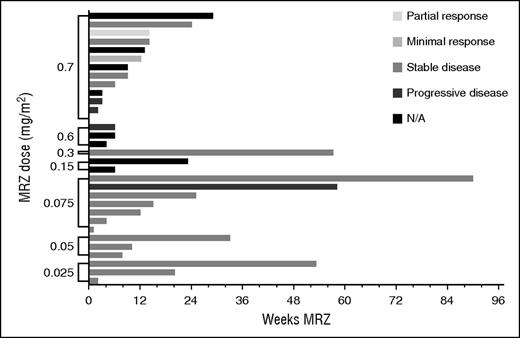
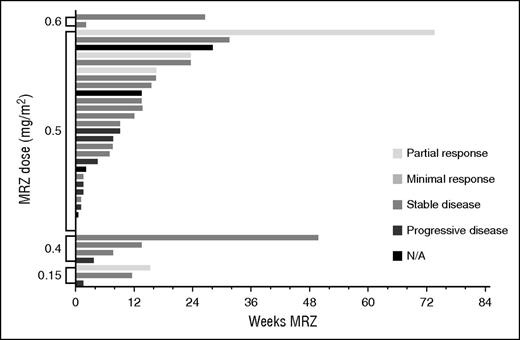
Total weeks of MRZ treatment received by patients are presented in Figures 1 and 2 (responses achieved are also displayed). More than 3 cycles of MRZ were received by 16 (50%) and 19 (53%) patients on schedules A and B, respectively. The mean (standard deviation) number of cycles completed in all dose groups was 4.4 cycles (4.98) and 3.5 cycles (4.16) on schedules A and B, respectively. The longest duration of treatment in a single patient was >23 cycles (630 days) on schedule A dose 0.075 mg/m2 and >19 cycles (515 days) on schedule B RP2D 0.5 mg/m2.
Total MRZ received and responses observed: patients on schedule A (weekly dosing). N/A, not applicable.
Total MRZ received and responses observed: patients on schedule A (weekly dosing). N/A, not applicable.
Total MRZ received and responses observed: patients on schedule B (twice-weekly dosing).
Total MRZ received and responses observed: patients on schedule B (twice-weekly dosing).
All patients in NPI-0052-101 Part 1 were off study as of January 13, 2014. Safety analyses included all 68 patients. Table 3 displays any treatment-emergent adverse events (TEAEs; defined as AEs that started after the initial dose of study drug or an existing AE that worsened during the study) that occurred in ≥20% of patients on either schedule. Table 4 displays TEAEs considered by the investigator(s) to be related to MRZ and that occurred in ≥10% of patients on either schedule. At least 1 related TEAE was experienced by 28 (88%) patients on schedule A, and 36 (100%) patients on schedule B. The 3 most common related TEAEs were the same in patients treated on both schedules: fatigue (41% and 52%, schedules A and B, respectively), nausea (41% and 36%), and headache (34% and 50%). There were 2 cardiac AEs considered to be possibly related to MRZ: 1 moderate (grade 2) AE of palpitations on schedule A 0.7 mg/kg and 1 mild (grade 1) AE of angina pectoris on schedule B RP2D 0.5 mg/m2; both AEs resolved. All grade 3 or higher AEs considered to be related to MRZ (also displayed in Table 4) were grade 3, except for 2 that were grade 4: increased blood creatinine (schedule A 0.075 mg/m2) and confusional state (schedule B RP2D 0.5 mg/m2). Grade 3–related AEs that occurred in >1 patient on either schedule included fatigue in 4 patients (2 schedule A, both at RP2D 0.7 mg/m2; 2 schedule B, 0.4 mg/m2 and RP2D 0.5 mg/m2) and thrombocytopenia in 4 patients (1 schedule A 0.7 mg/m2; 3 schedule B: 1 at 0.4 mg/m2, and 2 at RP2D 0.5 mg/m2).
Treatment-emergent adverse events in at least 20% of patients on either treatment schedule, by preferred term and frequency
| AE MedDRA preferred term . | Schedule A* (N = 32) . | Schedule B† (N = 36) . |
|---|---|---|
| Patients with ≥1 AE, n (%) | 30 (93.8) | 36 (100) |
| Fatigue | 16 (50.0) | 30 (83.3) |
| Headache | 16 (50.0) | 19 (52.8) |
| Nausea | 13 (40.6) | 17 (47.2) |
| Diarrhea | 11 (34.4) | 14 (38.9) |
| Dizziness | 9 (28.1) | 10 (27.8) |
| Upper respiratory tract infection | 8 (25.0) | 8 (22.2) |
| Constipation | 8 (25.0) | 9 (25.0) |
| Decreased appetite | 2 (6.3) | 9 (25.5) |
| Insomnia | 5 (15.6) | 13 (36.1) |
| Anemia | 5 (15.6) | 12 (33.3) |
| Edema peripheral | 3 (9.4) | 9 (25.0) |
| Pain in extremity | 2 (6.3) | 9 (25.0) |
| Pyrexia | 2 (6.3) | 8 (22.2) |
| AE MedDRA preferred term . | Schedule A* (N = 32) . | Schedule B† (N = 36) . |
|---|---|---|
| Patients with ≥1 AE, n (%) | 30 (93.8) | 36 (100) |
| Fatigue | 16 (50.0) | 30 (83.3) |
| Headache | 16 (50.0) | 19 (52.8) |
| Nausea | 13 (40.6) | 17 (47.2) |
| Diarrhea | 11 (34.4) | 14 (38.9) |
| Dizziness | 9 (28.1) | 10 (27.8) |
| Upper respiratory tract infection | 8 (25.0) | 8 (22.2) |
| Constipation | 8 (25.0) | 9 (25.0) |
| Decreased appetite | 2 (6.3) | 9 (25.5) |
| Insomnia | 5 (15.6) | 13 (36.1) |
| Anemia | 5 (15.6) | 12 (33.3) |
| Edema peripheral | 3 (9.4) | 9 (25.0) |
| Pain in extremity | 2 (6.3) | 9 (25.0) |
| Pyrexia | 2 (6.3) | 8 (22.2) |
Patients reporting more than one AE were counted once.
MedDRA, Medical Dictionary for Regulatory Activities v8.1.
Schedule A dosing was once weekly (days 1, 8, and 15 of 4-week cycles).
Schedule B dosing was twice weekly (days 1, 4, 8, and 11 of 3-week cycles); Schedule B patients could receive low-dose Dex.
Adverse events related to MRZ that occurred in 10% or more of patients on either treatment schedule, by preferred term and frequency, and all grade 3 or 4–related AEs
| AE MedDRA preferred term . | Related AEs . | Grade 3 or 4–related AEs . | |||
|---|---|---|---|---|---|
| Schedule A* (n = 32) . | Schedule B* (n = 36) . | All patients N = 68 . | Schedule A* (N = 32) . | Schedule B† (N = 36) . | |
| Patients with ≥1 related AE, n (%) | 28 (88%) | 36 (100%) | 64 (94%) | 0 | 0 |
| Fatigue | 13 (41%) | 19 (53%) | 32 (47%) | 2 (6%) | 2 (6%) |
| Headache | 11 (34%) | 18 (50%) | 29 (43%) | 0 | 0 |
| Nausea | 13 (41%) | 13 (36%) | 26 (38%) | 1 (3%) | 1 (3%) |
| Diarrhea | 8 (25%) | 11 (31%) | 19 (28%) | 1 (3%) | 0 |
| Dizziness | 9 (28%) | 9 (25%) | 18 (27%) | 1 (3%) | 0 |
| Vomiting | 10 (31%) | 7 (19%) | 17 (25%) | 1 (3%) | 1 (3%) |
| Hallucination | 4 (13%) | 4 (11%) | 8 (12%) | 0 | 0 |
| Decreased appetite | 2 (6%) | 6 (17%) | 8 (12%) | 0 | 0 |
| Anorexia | 5 (16%) | 2 (6%) | 7 (10%) | 0 | 0 |
| Confusional state | 2 (6%) | 5 (14%) | 7 (10%) | 1 (3%) | 1 (3%)‡ |
| Constipation | 4 (13%) | 3 (8%) | 7 (10%) | 0 | 0 |
| Insomnia | 4 (13%) | 3 (8%) | 7 (10%) | 0 | 1 (3%) |
| Anemia | 2 (6%) | 4 (11%) | 6 (9%) | 1 (3%) | 1 (3%) |
| Blood creatinine increased | 2 (6%) | 4 (11%) | 6 (9%) | 1 (3%)‡ | 1 (3%) |
| Dyspnea | 2 (6%) | 4 (11%) | 6 (9%) | 0 | 0 |
| Neuropathy peripheral | 1 (3%) | 5 (14%) | 6 (9%) | 0 | 1 (3%) |
| Asthenia | 1 (3%) | 4 (11%) | 5 (7%) | 0 | 0 |
| Gait disturbance | 1 (3%) | 4 (11%) | 5 (7%) | 0 | 0 |
| Pain in extremity | 1 (3%) | 4 (11%) | 4 (6%) | 0 | 0 |
| Mental status changes | 3 (9%) | 2 (6%) | 5 (7%) | 1 (3%) | 0 |
| Thrombocytopenia | 2 (6%) | 3 (8%) | 5 (7%) | 1 (3%) | 3 (8%) |
| Balance disorder | 1 (3%) | 3 (8%) | 4 (6%) | 1 (3%) | 0 |
| Alanine aminotransferase increased | 2 (6%) | 0 | 2 (3%) | 1 (3%) | 0 |
| Lymphopenia | 2 (6%) | 0 | 2 (3%) | 1 (3%) | 0 |
| Neutropenia | 1 (3%) | 1 (3%) | 2 (3%) | 0 | 1 (3%) |
| Vertigo | 2 (6%) | 0 | 2 (3%) | 1 (3%) | 0 |
| Febrile neutropenia | 0 | 1 (3%) | 1 (1%) | 0 | 1 (3%) |
| Hyponatremia | 0 | 1 (3%) | 1 (1%) | 0 | 1 (3%) |
| Neutrophil count decreased | 0 | 1 (3%) | 1 (1%) | 0 | 1 (3%) |
| Renal failure, acute | 1 (3%) | 0 | 1 (1%) | 1 (3%) | 0 |
| AE MedDRA preferred term . | Related AEs . | Grade 3 or 4–related AEs . | |||
|---|---|---|---|---|---|
| Schedule A* (n = 32) . | Schedule B* (n = 36) . | All patients N = 68 . | Schedule A* (N = 32) . | Schedule B† (N = 36) . | |
| Patients with ≥1 related AE, n (%) | 28 (88%) | 36 (100%) | 64 (94%) | 0 | 0 |
| Fatigue | 13 (41%) | 19 (53%) | 32 (47%) | 2 (6%) | 2 (6%) |
| Headache | 11 (34%) | 18 (50%) | 29 (43%) | 0 | 0 |
| Nausea | 13 (41%) | 13 (36%) | 26 (38%) | 1 (3%) | 1 (3%) |
| Diarrhea | 8 (25%) | 11 (31%) | 19 (28%) | 1 (3%) | 0 |
| Dizziness | 9 (28%) | 9 (25%) | 18 (27%) | 1 (3%) | 0 |
| Vomiting | 10 (31%) | 7 (19%) | 17 (25%) | 1 (3%) | 1 (3%) |
| Hallucination | 4 (13%) | 4 (11%) | 8 (12%) | 0 | 0 |
| Decreased appetite | 2 (6%) | 6 (17%) | 8 (12%) | 0 | 0 |
| Anorexia | 5 (16%) | 2 (6%) | 7 (10%) | 0 | 0 |
| Confusional state | 2 (6%) | 5 (14%) | 7 (10%) | 1 (3%) | 1 (3%)‡ |
| Constipation | 4 (13%) | 3 (8%) | 7 (10%) | 0 | 0 |
| Insomnia | 4 (13%) | 3 (8%) | 7 (10%) | 0 | 1 (3%) |
| Anemia | 2 (6%) | 4 (11%) | 6 (9%) | 1 (3%) | 1 (3%) |
| Blood creatinine increased | 2 (6%) | 4 (11%) | 6 (9%) | 1 (3%)‡ | 1 (3%) |
| Dyspnea | 2 (6%) | 4 (11%) | 6 (9%) | 0 | 0 |
| Neuropathy peripheral | 1 (3%) | 5 (14%) | 6 (9%) | 0 | 1 (3%) |
| Asthenia | 1 (3%) | 4 (11%) | 5 (7%) | 0 | 0 |
| Gait disturbance | 1 (3%) | 4 (11%) | 5 (7%) | 0 | 0 |
| Pain in extremity | 1 (3%) | 4 (11%) | 4 (6%) | 0 | 0 |
| Mental status changes | 3 (9%) | 2 (6%) | 5 (7%) | 1 (3%) | 0 |
| Thrombocytopenia | 2 (6%) | 3 (8%) | 5 (7%) | 1 (3%) | 3 (8%) |
| Balance disorder | 1 (3%) | 3 (8%) | 4 (6%) | 1 (3%) | 0 |
| Alanine aminotransferase increased | 2 (6%) | 0 | 2 (3%) | 1 (3%) | 0 |
| Lymphopenia | 2 (6%) | 0 | 2 (3%) | 1 (3%) | 0 |
| Neutropenia | 1 (3%) | 1 (3%) | 2 (3%) | 0 | 1 (3%) |
| Vertigo | 2 (6%) | 0 | 2 (3%) | 1 (3%) | 0 |
| Febrile neutropenia | 0 | 1 (3%) | 1 (1%) | 0 | 1 (3%) |
| Hyponatremia | 0 | 1 (3%) | 1 (1%) | 0 | 1 (3%) |
| Neutrophil count decreased | 0 | 1 (3%) | 1 (1%) | 0 | 1 (3%) |
| Renal failure, acute | 1 (3%) | 0 | 1 (1%) | 1 (3%) | 0 |
Patients reporting more than one AE were counted once.
MedDRA, Medical Dictionary for Regulatory Activities v8.1.
Schedule A dosing was once weekly (days 1, 8, and 15 of 4-week cycles).
Schedule B dosing was twice weekly (days 1, 4, 8, and 11 of 3-week cycles); schedule B patients could receive low-dose Dex.
Grade 4 event.
Tables 5 and 6 display DLTs that occurred on study. Five patients on schedule A experienced AEs that were considered DLTs by protocol at the time of occurrence (renal failure acute, blood creatinine increased ×2, fatigue ×2, hallucination, mental status changes, balance disorder, and confusional state); 4 patients on schedule B experienced DLTs (paranoia, gait disturbance, nausea, and confusional state).
Schedule A DLTs
| Patient's starting dose cohort . | Dose when DLT occurred . | AE recorded as DLT . | Was the AE serious? . | Severity . | Relationship to MRZ/Dex . | Action taken with MRZ/Dex . | Outcome . |
|---|---|---|---|---|---|---|---|
| 0.075 mg/m2 * | 0.075 mg/m2 | Renal failure acute | Yes | Grade 3 | Possible | MRZ discontinued | Resolved |
| Blood creatinine increased | Yes | Grade 4 | Possible | MRZ discontinued | resolved | ||
| Blood creatinine increased | Yes | Grade 3 | Possible | MRZ discontinued | Resolved | ||
| 0.7 mg/m2 | 0.6 mg/m2 | Nausea | Yes | Grade 3 | Possible | MRZ dose reduced | Resolved |
| Vomiting | Yes | Grade 3 | Possible | MRZ dose reduced | Resolved | ||
| 0.7 mg/m2 | 0.6 mg/m2 | Fatigue | No | Grade 3 | Possible | MRZ discontinued | Resolved |
| 0.7 mg/m2 | 0.7 mg/m2 | Hallucinations | No | Grade 2 | Possible | MRZ dose reduced | Resolved |
| 0.7 mg/m2 | 0.7 mg/m2 | Mental status changes | Yes | Grade 3 | Possible | none | Resolved |
| Balance disorder | Yes | Grade 3 | Possible | none | Resolved | ||
| RP2D 0.7 mg/m2 † | 0.7 mg/m2 | Confusional state | Yes | Grade 3 | Possible | MRZ discontinued | Resolved |
| Patient's starting dose cohort . | Dose when DLT occurred . | AE recorded as DLT . | Was the AE serious? . | Severity . | Relationship to MRZ/Dex . | Action taken with MRZ/Dex . | Outcome . |
|---|---|---|---|---|---|---|---|
| 0.075 mg/m2 * | 0.075 mg/m2 | Renal failure acute | Yes | Grade 3 | Possible | MRZ discontinued | Resolved |
| Blood creatinine increased | Yes | Grade 4 | Possible | MRZ discontinued | resolved | ||
| Blood creatinine increased | Yes | Grade 3 | Possible | MRZ discontinued | Resolved | ||
| 0.7 mg/m2 | 0.6 mg/m2 | Nausea | Yes | Grade 3 | Possible | MRZ dose reduced | Resolved |
| Vomiting | Yes | Grade 3 | Possible | MRZ dose reduced | Resolved | ||
| 0.7 mg/m2 | 0.6 mg/m2 | Fatigue | No | Grade 3 | Possible | MRZ discontinued | Resolved |
| 0.7 mg/m2 | 0.7 mg/m2 | Hallucinations | No | Grade 2 | Possible | MRZ dose reduced | Resolved |
| 0.7 mg/m2 | 0.7 mg/m2 | Mental status changes | Yes | Grade 3 | Possible | none | Resolved |
| Balance disorder | Yes | Grade 3 | Possible | none | Resolved | ||
| RP2D 0.7 mg/m2 † | 0.7 mg/m2 | Confusional state | Yes | Grade 3 | Possible | MRZ discontinued | Resolved |
As of protocol amendment 4, >60 patients with similar baseline renal function had been treated at MRZ doses as high as 0.7 mg/m2 (nearly 10-fold higher) on an identical schedule without DLT or demonstration of nephrotoxicity. Therefore, when the dose of 0.075 mg/m2 was shown to be safe in a cohort of 6 new patients (without observation of DLT), the protocol permitted dose escalation to continue using dose doubling in cohorts of 1-3 patients evaluable for toxicity, until observation of grade 2 or greater drug-related toxicity. Thereafter, dose escalation increments were limited to <50% in groups of at least 3 patients evaluation for toxicity until observation of DLT.
Schedule A of this study was conducted in parallel with separate MRZ trials (NPI-0052-100 and NPI-0052-102) that studied doses of 0.8 and 0.9 mg/m2 at the same schedule and found that MRZ, administered on days 1, 8, and 15, was generally well tolerated through 0.7 mg/m2, and therefore, the parallel-run trials influenced the designation of the 0.7-mg/m2 dose as the RP2D of schedule A.
Schedule B investigator-identified and sponsor-assessed DLTs
| Patient’s starting dose cohort . | Dose when DLT occurred . | AE recorded as DLT . | Was the AE serious? . | Severity . | Relationship to MRZ/Dex . | Action taken with MRZ /Dex . | Outcome . |
|---|---|---|---|---|---|---|---|
| RP2D 0.5 mg/m2 | 0.5 mg/m2 | Nausea | Yes | Grade 3 | MRZ probable/Dex not related | None | Resolved |
| RP2D 0.5 mg/m2 | 0.5 mg/m2 | Confusional state | Yes | Grade 4 | MRZ probable/Dex not related | MRZ discontinued | Resolved |
| 0.6 mg/m2 | 0.4 mg/m2 | Gait disturbance | No | Grade 2 | MRZ probable/Dex not related | MRZ dose reduced, dose delayed | Resolved |
| 0.6 mg/m2 | 0.6 mg/m2 | Paranoia | No | Grade 2 | MRZ possible/Dex not related | MRZ discontinued | Resolved |
| Patient’s starting dose cohort . | Dose when DLT occurred . | AE recorded as DLT . | Was the AE serious? . | Severity . | Relationship to MRZ/Dex . | Action taken with MRZ /Dex . | Outcome . |
|---|---|---|---|---|---|---|---|
| RP2D 0.5 mg/m2 | 0.5 mg/m2 | Nausea | Yes | Grade 3 | MRZ probable/Dex not related | None | Resolved |
| RP2D 0.5 mg/m2 | 0.5 mg/m2 | Confusional state | Yes | Grade 4 | MRZ probable/Dex not related | MRZ discontinued | Resolved |
| 0.6 mg/m2 | 0.4 mg/m2 | Gait disturbance | No | Grade 2 | MRZ probable/Dex not related | MRZ dose reduced, dose delayed | Resolved |
| 0.6 mg/m2 | 0.6 mg/m2 | Paranoia | No | Grade 2 | MRZ possible/Dex not related | MRZ discontinued | Resolved |
Two patients died within 28 days of the last dose of MRZ. One schedule A patient died due to subdural hematoma 10 days after 1 dose of MRZ 0.7 mg/m2 (baseline and day 2 platelets: 236 and 146(L) K/mm3); the investigator assessed the event as related not to MRZ but to a previously existing comorbid condition. One schedule B patient died due to pneumonia 19 days after the last dose of MRZ 0.4 mg/m2 after receiving 5 cycles of MRZ and receiving Dex in cycles 4 and 5; baseline and cycle 5 neutrophils were 5.05 and 5.11 K/mm3; the investigator assessed the event as not related to MRZ.
No difference in toxicity profile was expected between the 2 formulations (liquid: 0.1 mg/mL in 40% propylene glycol, 50% citrate buffer, and 10% ethanol; lyophile: 0.2 mg/mL in 55% propylene glycol, 40% citrate buffer, 5% ethanol, and 6 mg/mL sucrose). No substantial difference in toxicity profile was observed in patients receiving the lyophile formulation compared with the liquid formulation used in schedule A. The lyophile formulation was selected for schedule B due to the advantages of the new drug product formulation including simplified pharmacy preparation, longer stability following reconstitution, and half the volume delivered.
Efficacy
Efficacy response data were evaluated for all patients. Figures 1 and 2 display the response achieved by each patient in correlation to the weeks of MRZ treatment. An IMWG response of MR or better was seen in 2 out of 32 patients (6%) on schedule A and 4 out of 36 patients (11%) on schedule B. PRs included 1 patient at the schedule A 0.7 mg/m2 dose (RP2D), 1 patient at schedule B 0.15 mg/m2, and 3 patients at schedule B 0.5 mg/m2 (RP2D; these 3 patients received concomitant Dex). These responses were in patients who had received prior BZ, lenalidomide, and/or thalidomide.
Discussion
MRZ is a novel, irreversible, pan-subunit proteasome inhibitor in clinical development for the treatment of relapsed or RRMM. Proteasome inhibitors have been validated as effective therapy for patients with relapsed or RRMM based on the approvals of BZ and CFZ in multiple regions. Proteasome inhibitors are used in combination with Dex and may be combined with an IMiD, such as thalidomide, lenalidomide, or pomalidomide, or chemotherapeutic agents including cyclophosphamide or melphalan.35
The primary purpose of this open-label, 3+3 dose-escalation study was to determine the MTD for MRZ given IV either weekly or twice weekly in relapsed or RRMM patients. Secondary end points included determination of an RP2D and evaluation of the safety, toxicity, and PK profiles. A total of 68 patients were evaluated across 2 dosing schedules and 9 dose levels between 0.025 and 0.7 mg/m2. Schedule A of this study was conducted in parallel with separate MRZ trials (NPI-0052-100 and NPI-0052-102) that studied doses of 0.8 and 0.9 mg/m2 at the same schedule and found that MRZ was generally well tolerated through 0.7 mg/m2. Therefore, the parallel-run trials influenced the designation of the RP2D on schedule A as 0.7 mg/m2 infused over 10 minutes. The RP2D on schedule B was determined to be 0.5 mg/m2 infused over 2 hours (this dose was also identified in study NPI-0052-102).
The RRMM patients enrolled in this trial were previously treated patients who had received an average of 4.9 and 7.3 prior treatment regimens on schedules A and B, respectively. Prior BZ was received by 69% of patients on schedule A and by 97% on schedule B, and almost all patients had received a prior IMiD.
Although this was a phase 1 study with a primary objective to determine the MTD of MRZ IV in RRMM, MRZ clinical activity was suggested in patients who received ≥1 MRZ dose: 2 of 32 (6%) schedule A patients achieved MR or better (both at RP2D 0.7 mg/m2), and 4 of 36 (11%) schedule B patients achieved MR or better (1 at 0.15 mg/m2 and 3 at RP2D 0.5 mg/m2).
Patients tolerated lengthy exposure times on both the weekly and twice-weekly dosing schedules employed in the trial (shown in Figures 1 and 2). The mean duration of treatment on schedule A was 126 days (range 1-630) and on schedule B was 91 days (range 1-515). The mean number of cycles on study was 2.5 cycles (range 0-6) and 3.6 cycles (range 0-19) for the 13 patients treated at the schedule A RP2D, and the 24 patients treated at the schedule B RP2D. The longest time a patient remained on schedule A was >23 cycles (dose of 0.075 mg/m2) and on schedule B was >19 cycles (RP2D 0.5 mg/m2).
In this phase 1 study of 68 patients, this natural product β lactone MRZ (an irreversible inhibitor of the 3 proteasome subunits) displayed evidence of a different safety profile from other synthetic proteasome inhibitors.2 The most common (>20% of patients) related AEs observed in patients treated with MRZ on either dosing schedule (schedule A or B) included fatigue, headache, nausea, diarrhea, dizziness, and vomiting. Significant peripheral neuropathy was not noted, unlike in BZ-containing regimens,2,9 where particularly painful PN may occur. There were only 2 possibly drug-related cardiac AEs observed (moderate palpitations, schedule A 0.7 mg/kg; mild angina pectoris, schedule B RP2D 0.5 mg/m2; both resolved); this may be an encouraging result, as studies have shown cardiac dysfunction following use of BZ and CFZ.14,36-38 Hematologic toxicity appeared mild compared with that reported with BZ and CFZ: grade 3 thrombocytopenia considered to be possibly related to MRZ was seen in 1 out of 32 (6%) schedule A patients and 3 out of 36 (8%) schedule B patients, whereas rates of 16% to 30% and 11% to 32% have been reported in single agent trials of BZ and CFZ, respectively.39 CNS toxicities were encountered when MRZ was administered over short infusion times (1 or 10 minutes) at higher doses (>0.5 mg/m2). Infusion time was increased to 2 hours in response to separate MRZ trials where the increased infusion time appeared to ameliorate the potential for CNS AEs.
PK data (to be reported separately) confirmed findings from previous clinical studies.40,41 MRZ concentrations peaked shortly after infusion and the drug had a short plasma half-life. High estimates of volume of distribution and clearance across the dose range examined were consistent between days 1 and 15, indicating wide tissue distribution and/or binding to blood components and suggesting involvement of extrahepatic clearance mechanisms. Pharmacodynamic analyses (to be reported separately) showed that partial or complete inhibition of the 3 proteasome subunits in packed whole blood could be achieved with either once-weekly or twice-weekly MRZ dosing.
Collectively, MRZ’s inhibition of the 3 separate enzyme activities of isolated proteasomes, and its blocking of the 3 proteasome subunits (CT-L, T-L, and C-L), may differentiate MRZ from other proteasome inhibitors. Both of the RP2Ds in this study (0.7 mg/m2 infused over 10 minutes once weekly and 0.5 mg/m2 infused over 2 hours twice weekly) were generally well tolerated and offered signals of clinical activity. Clinical benefit responses (MR or better) were achieved in 6 patients, including 5 PRs: 1 on schedule A and 4 on schedule B. The regimen of choice for further development in RRMM is the schedule B RP2D of MRZ (0.5 mg/m2 on days 1, 4, 8, and 11 in 3-week cycles infused over 2 hours with Dex given on the day of and the day after MRZ dosing); 3 of the 5 PRs occurred in patients receiving this treatment regimen. The majority of patients in the study had received ≥2 cycles of prior therapy with either BZ or an IMiD, and disease response in these subsets (based on IMWG Uniform Response Criteria) was comparable to the safety population, suggesting that MRZ may be active in patients who have already received standard-of-care therapy.
Years of BZ use have elicited evidence of acquired resistance or toxicity to the proteasome inhibitor and present the basis and rationale for the development of second-generation proteasome inhibitors.17 This study highlights that proteasome inhibitor MRZ was generally well tolerated and may suggest clinical activity in previously treated relapsed MM or RRMM patients. Further clinical development with MRZ is justified in view of the established validation of the proteasome target and the mechanistic differences between proteasome inhibitors. MRZ provides a valid platform for combinatorial studies, especially with immunomodulatory drugs, such as pomalidomide, in the future.17,23,24,42
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Mike Paladino and Alison Hannah for their contributions to the research, Michelle Hare and Francis Burrows for their assistance with writing this manuscript, and Michelle Maglio for editorial support. The authors also gratefully acknowledge the research nurses, clinical study coordinators, and participating patients and their families.
Authorship
Contribution: P.G.R., T.M.Z., C.C.H., M. Talpaz, A.A.C.-K., J.L.K., J.P.L., D.C., A.J.J., and K.C.A. were involved in enrolling patients and reviewed the data; S.R. was the medical monitor and contributed to the data analysis and writing; and M. Trikha contributed to data analysis and writing.
Conflict-of-interest disclosure: K.C.A. is on the advisory boards of Bristol-Myers Squibb Pharmaceuticals, Celgene Corporation, Gilead Pharmaceuticals, and Millenium (The Takeda Oncology Company) and is the scientific founder of Acetylon Pharmaceutcials and OncoPep, Inc. D.C. is a consultant for Triphase Accelerator Corp. A.J.J. is a consultant for and on the advisory boards of Amgen Inc., Bristol-Myers Squibb Pharmaceuticals, Celgene Corporation, Janssen Pharmaceuticals, Karyopharm Therapeutics Inc., Millenium (The Takeda Oncology Company), SkylineDx, and Sanofi-Aventis Pharmaceuticals. J.L.K. is a member of the Data Monitoring Committee for Incyte Corporation and Pharmacyclics, is a consultant for Onyx Pharmaceuticals and Millenium (The Takeda Oncology Company), and receives research funding from Celgene Corporation, Merck & Co., Novartis Pharmaceuticals, and Onyx Pharmaceuticals. J.P.L. receives research funding from the Celgene Corporation, Millenium (The Takeda Oncology Company), Novartis Pharmaceuticals, and Onyx Pharmaceuticals and is a consultant for Novartis Pharmaceuticals. S.R. received payment from Triphase Accelerator Corp. P.G.R. serves on advisory committes of and receives research funding from Celgene Corporation and Millenium (The Takeda Oncology Company). M. Trikha is employed by and received payment from the Triphase Accelerator Corp. The remaining authors declare no competing financial interests.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 232, Boston, MA 02215; e-mail: paul_richardson@dfci.harvard.edu.