Key Points
ATRA and FLT3 TKIs have synergistic activity against FLT3/ITD+ AML cell lines and patient samples.
Combination reduces the leukemia stem cell population and improves survival in genetic and xenograft AML mouse models.
Abstract
FMS-like tyrosine kinase 3 (FLT3)-mutant acute myeloid leukemia (AML) portends a poor prognosis, and ineffective targeting of the leukemic stem cell (LSC) population remains one of several obstacles in treating this disease. All-trans retinoic acid (ATRA) has been used in several clinical trials for the treatment of nonpromyelocytic AML with limited clinical activity observed. FLT3 tyrosine kinase inhibitors (TKIs) used as monotherapy also achieve limited clinical responses and are thus far unable to affect cure rates in AML patients. We explored the efficacy of combining ATRA and FLT3 TKIs to eliminate FLT3/internal tandem duplication (ITD)+ LSCs. Our studies reveal highly synergistic drug activity, preferentially inducing apoptosis in FLT3/ITD+ cell lines and patient samples. Colony-forming unit assays further demonstrate decreased clonogenicity of FLT3/ITD+ cells upon treatment with ATRA and TKI. Most importantly, the drug combination depletes FLT3/ITD+ LSCs in a genetic mouse model of AML, and prolongs survival of leukemic mice. Furthermore, engraftment of primary FLT3/ITD+ patient samples is reduced in mice following treatment with FLT3 TKI and ATRA in combination, with evidence of cellular differentiation occurring in vivo. Mechanistically, we provide evidence that the synergism of ATRA and FLT3 TKIs is at least in part due to the observation that FLT3 TKI treatment upregulates the antiapoptotic protein Bcl6, limiting the drug’s apoptotic effect. However, cotreatment with ATRA reduces Bcl6 expression to baseline levels through suppression of interleukin-6 receptor signaling. These studies provide evidence of the potential of this drug combination to eliminate FLT3/ITD+ LSCs and reduce the rate of relapse in AML patients with FLT3 mutations.
Introduction
Acute myeloid leukemia (AML) is an aggressive bone marrow (BM) malignancy. FMS-like tyrosine kinase 3 (FLT3) is one of the most commonly mutated genes in AML with approximately one-third of AML patients affected, mostly in the form of internal tandem duplication (ITD) mutations which confer a very poor prognosis.1-7 To improve the cure rate for FLT3-mutant AML, a number of FLT3 tyrosine kinase inhibitors (TKIs) have been developed to inhibit FLT3 signaling.8 Although several recent FLT3 TKIs are proving increasingly successful at achieving high levels of FLT3 inhibition, monotherapy is unlikely to cure FLT3-mutated AML patients.8 This is likely due to the fact that FLT3 mutations are only 1 of an estimated 10 or more genetic alterations that are present in each case of AML.9 The compendium of altered genes in this disease is large and thus the development of molecularly targeted therapy for each of these mutations is a daunting task. Fortunately, many of these mutations are likely to funnel into a more limited number of signaling pathways that are amenable to drug targeting.10 Combining direct FLT3 inhibition with drugs that also inhibit these critical pathways holds promise for greatly improving the cure rate in patients with FLT3-mutant leukemias.
Accumulating evidence supports the idea that leukemias are clonal disorders originating in 1 or a few primitive multipotential hematopoietic cell(s). A very small fraction of leukemia cells, called leukemia stem cells (LSCs), possess characteristics associated with normal hematopoietic stem cells (HSCs), specifically the ability to self-renew and differentiate (to a limited degree) to maintain and propagate the leukemia cell pool.11 FLT3 mutations often occur late in the clonal evolution of AML, following accumulation of early alterations in genomic landscaping genes such as DNMT3A, IDH1/2, and NPM1 in preleukemic HSC clones.12 However, there remains compelling evidence that FLT3 mutations are present in the LSC fraction as defined by the ability of the cells to propagate the disease in immunodeficient mice. Of note, ITD mutations of FLT3 have been shown to be present in the lineage-negative CD34+/CD38− LSC fraction of primary AML samples at the same allelic ratio as unsorted cells, and in most cases this sorted LSC population is able to engraft NOD-SCID mice.13 Other investigators have demonstrated that FLT3/ITD is present in the CD34+/CD33− population in nearly 80% of cases harboring the mutation at diagnosis, and the presence of the mutation within this primitive population portends a poor prognosis.14 In order for any AML therapy to be curative, it needs to be effective against not only the bulk leukemia cells but against the LSCs as well. Data have also shown the importance of several “stem cell” pathways in maintaining LSCs in AML, including the retinoic acid, WNT/β-catenin, Notch, and Hedgehog (Hh) pathways.15-18 Some of these pathways have already been shown to be active specifically in FLT3-mutant AML whereas others have been shown to be active broadly in AML.15-20 Each of these pathways are candidates for combination therapy with FLT3 inhibitors.
Retinoic acid (RA) has been shown to play an important role in the differentiation of normal HSCs, and treatment with all-trans RA (ATRA) has greatly improved the cure rate for acute promyelocytic leukemia (APL).21 Stimulation of the RA pathway with ATRA has also been tried as monotherapy for the treatment of nonpromyelocytic AML patients and has shown some clinical activity, although it has not been replicated in all studies.15,22-25 Our hypothesis is that LSCs may be susceptible to treatment with ATRA and, when combined with FLT3 inhibition, these drugs will be able to overcome the block in differentiation as well as negate the strong proliferative and survival signaling characteristic of FLT3 mutations. Interestingly, APL patients expressing FLT3/ITD mutations are successfully treated with ATRA combined with chemotherapy and/or arsenic trioxide.26,27 Moreover, previous studies have reported on the combinatorial effect of ATRA and FLT3 inhibitors on apoptosis in FLT3/ITD+ cell lines.28,29 We therefore explored the combination of molecularly targeting the RA pathway together with FLT3 TKIs to determine the effect on FLT3-mutant LSCs.
Methods
Growth inhibition
Cells were seeded at a density of 1 × 105 to 2.5 × 105 cells per mL in the presence or absence of compounds for the indicated times. Cell proliferation was measured in quadruplicate using the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) assay according to the manufacturer’s instructions (Roche Applied Science). Viable cell counts were performed by Trypan blue exclusion at 24-hour intervals. For colony-forming unit (CFU) assays, cells were plated at a density of 5 × 102 to 2 × 104 cells per mL in methylcellulose (Methocult H4230, H4435, or M3434; Stem Cell Technologies) and incubated at 37°C. Total colony counts and/or burst-forming unit erythroid, CFU-granulocyte/macrophage, CFU-granulocyte, and CFU-macrophage counts were obtained after 7 to 14 days.
Transplantation experiments
Transplantation of Molm14, FLT3/ITD+ primary patient sample, and leukemic NHD13;FLT3/ITD BM cells was performed as described previously.30-32 For details on transplantation, drug administration, and experimental analysis, see supplemental Methods (available on the Blood Web site).
Bcl6 knockdown
Bcl6 knockdown was performed using Expression Arrest TRIPZ lentiviral shRNAmir constructs (Thermo Scientific) as previously described.33 For details on lentiviral transduction and short hairpin RNA (shRNA) induction, see supplemental Methods.
Statistical analysis
Statistical analysis was performed with the Student unpaired 2-tailed t test and log-rank test by use of the GraphPad software analysis program (Prism). P values of <.05 were considered to be statistically significant. All data are presented as the mean ± standard deviation (SD).
Results
Synergistic effects of FLT3 TKIs and ATRA in FLT3/ITD+ cell lines and primary AML samples
In order to assess the effect of combination therapy, human FLT3/ITD+ AML cell lines (Molm14 and MV4-11), along with FLT3 wild-type (FLT3/WT) cell lines where there is no active FLT3 signaling (THP-1, HL60, and NB4), were treated with increasing concentrations of FLT3 TKI alone (sorafenib, AC220, or TTT-3002), ATRA alone, or FLT3 TKI plus ATRA (combination) (Table 1; Figure 1A-C; supplemental Figure 1). Proliferation of the FLT3/ITD+ cell lines is sensitive to sorafenib, AC220, and TTT-3002 with 50% inhibitory concentration values of 5, 1.5, and 0.5 nM, respectively, whereas there is no antiproliferative effect on the FLT3/WT cell lines.31 MV4-11 and THP-1 cells are moderately sensitive to ATRA alone, with an 50% inhibitory concentration of 100 nM, although the other cell lines do not demonstrate a change in rate of proliferation (supplemental Figure 1). The combinatorial effect on cell proliferation was assessed by calculation of combinatorial index (CI) values across a range of drug concentrations using the Chou-Talalay method.34 Synergistic effects were observed for the combination of ATRA with FLT3 TKIs against FLT3/ITD+ cells, with CI values of 0.1 to 0.7, whereas no synergistic effects were observed for FLT3/WT cells, with CI values >1.0 (Table 1; Figure 1A-C). A reduction in viable cell counts was also observed in FLT3/ITD+ AML patient samples when exposed to combination treatment (Figure 1D; supplemental Table 1).
CI values for FLT3 TKIs in combination with ATRA
| Cell line . | Sorafenib . | AC220 . | TTT-3002 . | |||
|---|---|---|---|---|---|---|
| CI (EC50) . | CI (EC90) . | CI (EC50) . | CI (EC90) . | CI (EC50) . | CI (EC90) . | |
| Molm14 | 0.32 | 0.46 | 0.48 | 0.68 | 0.53 | 0.47 |
| MV4-11 | 0.18 | 0.38 | 0.14 | 0.62 | 0.04 | 0.03 |
| Cell line . | Sorafenib . | AC220 . | TTT-3002 . | |||
|---|---|---|---|---|---|---|
| CI (EC50) . | CI (EC90) . | CI (EC50) . | CI (EC90) . | CI (EC50) . | CI (EC90) . | |
| Molm14 | 0.32 | 0.46 | 0.48 | 0.68 | 0.53 | 0.47 |
| MV4-11 | 0.18 | 0.38 | 0.14 | 0.62 | 0.04 | 0.03 |
CI values obtained by MTT assay at 48 hours, as in Figure 1, across a range of concentrations of ATRA (0-100 nM), sorafenib (0-20 nM), AC220 (0-10 nM), and TTT-3002 (0-5 nM) in equimolar ratios.
EC50, half maximal effective concentration; EC90, 90% effective concentration.
FLT3 TKIs synergize with ATRA to reduce proliferation of FLT3/ITD cells. (A-C) FLT3/ITD+ Molm14 cells were treated with (A) sorafenib (0-20 nM), (B) AC220 (0-10 nM), or (C) TTT-3002 (0-5 nM) either alone or in combination with ATRA (0-100 nM) for 48 hours, and cell proliferation was measured in quadruplicate by MTT assay. Error bars indicate average percentage OD ± SD. CI values were calculated by the Chou-Talalay method, shown at right. The dashed line designates a CI value of 1, with CI <1 being synergistic, CI = 1 being additive, and CI >1 being antagonistic. Data are representative of 3 independent experiments. (D) Viable leukemic blasts were isolated from FLT3/ITD+ AML patients and treated with sorafenib (20 nM) and/or ATRA (100 nM) in vitro. Viable cell counts measured by Trypan blue exclusion staining in triplicate at 72 hours. Error bars indicate average ± SD, and significant P values relative to combination treatment are shown (*P < .05, **P < .01, ***P < .001). OD, optical density.
FLT3 TKIs synergize with ATRA to reduce proliferation of FLT3/ITD cells. (A-C) FLT3/ITD+ Molm14 cells were treated with (A) sorafenib (0-20 nM), (B) AC220 (0-10 nM), or (C) TTT-3002 (0-5 nM) either alone or in combination with ATRA (0-100 nM) for 48 hours, and cell proliferation was measured in quadruplicate by MTT assay. Error bars indicate average percentage OD ± SD. CI values were calculated by the Chou-Talalay method, shown at right. The dashed line designates a CI value of 1, with CI <1 being synergistic, CI = 1 being additive, and CI >1 being antagonistic. Data are representative of 3 independent experiments. (D) Viable leukemic blasts were isolated from FLT3/ITD+ AML patients and treated with sorafenib (20 nM) and/or ATRA (100 nM) in vitro. Viable cell counts measured by Trypan blue exclusion staining in triplicate at 72 hours. Error bars indicate average ± SD, and significant P values relative to combination treatment are shown (*P < .05, **P < .01, ***P < .001). OD, optical density.
Sorafenib and ATRA induce apoptosis in FLT3/ITD+ cells
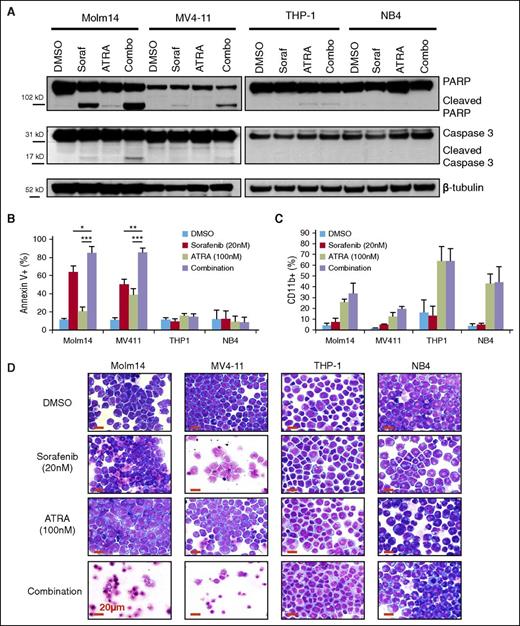
To further explore the synergistic effect of combining FLT3 TKI and ATRA treatment in FLT3/ITD+ leukemia cells, assays of apoptosis and differentiation were performed on leukemia cell lines. A fixed concentration of each drug (20 nM sorafenib and 100 nM ATRA) was chosen to assess the combinatorial effect on leukemia cells. Western blotting demonstrated increased activation of proapoptotic pathways after 24-hour treatment with sorafenib and ATRA and it was restricted to FLT3/ITD+ AML cell lines (Figure 2A). Annexin V staining revealed an increased induction of apoptosis when FLT3/ITD+ cells are treated with sorafenib plus ATRA compared with either drug alone (Figure 2B). Similar results were recorded for AC220 and TTT-3002 in combination with ATRA, providing further evidence that the effects are due to FLT3 inhibition rather than an off-target effect of sorafenib (supplemental Figure 2A-B). Synergistic CI values for induction of apoptosis of FLT3/ITD+ cell lines following treatment over a range of drug concentrations were 0.33 to 0.52 for Molm14 cells and 0.07 to 0.11 for MV4-11 cells (supplemental Figure 3). In contrast, there were no combinatorial effects for FLT3/WT cell lines (Figure 2A-B; supplemental Figure 2). Furthermore, there was no combinatorial effect on cell differentiation as indicated by upregulation of CD11b expression, with ATRA alone being equally efficacious to combination treatment at 72 hours (Figure 2C). Evidence of differentiation by morphologic assessment is present in both FLT3/WT and FLT3/ITD+ ATRA-treated cells, whereas an increase in cell death is observed following sorafenib or sorafenib plus ATRA treatment only in FLT3/ITD+ cells (Figure 2D).
Sorafenib plus ATRA induces apoptosis in FLT3/ITD cell lines. FLT3/ITD+ (Molm14, MV4-11) and FLT3/WT (THP-1, NB4) leukemia cell lines were treated with sorafenib (Soraf, 20 nM) and/or ATRA (100 nM). (A) Expression of caspase 3 and PARP cleavage by western blotting of cell lysates at 24 hours. (B) AnnexinV binding at 48 hours, represented by average of 3 independent experiments. Error bars indicate average ± SD (*P < .05, **P < .01, ***P < .001). (C) CD11b expression by flow cytometry at 72 hours, represented by average of 3 independent experiments. Error bars indicate average ± SD. (D) Cellular morphology at 72 hours by Wright-Giemsa staining, magnification ×50. Increased levels of cell death are observed in sorafenib- and combination-treated FLT3/ITD+ cells, whereas evidence of differentiation is apparent in ATRA-treated cells. Red scale bar represents 20 µm. PARP, poly (ADP-ribose) polymerase.
Sorafenib plus ATRA induces apoptosis in FLT3/ITD cell lines. FLT3/ITD+ (Molm14, MV4-11) and FLT3/WT (THP-1, NB4) leukemia cell lines were treated with sorafenib (Soraf, 20 nM) and/or ATRA (100 nM). (A) Expression of caspase 3 and PARP cleavage by western blotting of cell lysates at 24 hours. (B) AnnexinV binding at 48 hours, represented by average of 3 independent experiments. Error bars indicate average ± SD (*P < .05, **P < .01, ***P < .001). (C) CD11b expression by flow cytometry at 72 hours, represented by average of 3 independent experiments. Error bars indicate average ± SD. (D) Cellular morphology at 72 hours by Wright-Giemsa staining, magnification ×50. Increased levels of cell death are observed in sorafenib- and combination-treated FLT3/ITD+ cells, whereas evidence of differentiation is apparent in ATRA-treated cells. Red scale bar represents 20 µm. PARP, poly (ADP-ribose) polymerase.
Modulation of Bcl6 by FLT3 TKIs and ATRA
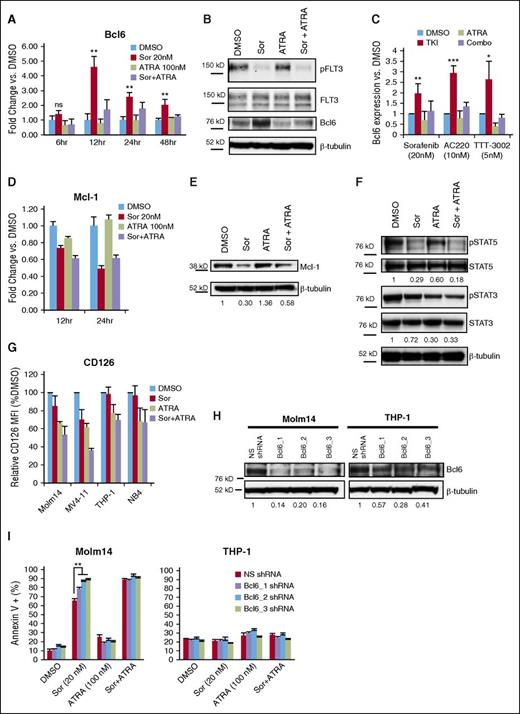
Based on the strong apoptotic effect observed for combined treatment with FLT3 TKIs and ATRA in FLT3/ITD+ cells, we further investigated the molecular mechanism underlying the drug synergy. Inhibition of BCR-ABL1 in chronic myeloid leukemia and acute lymphoblastic leukemia by several TKIs has been previously shown to lead to upregulation of B-cell lymphoma 6 (Bcl6), thereby partially negating the apoptotic effect of the drugs via transcriptional inactivation of the p53 pathway.35,36 We hypothesized that FLT3-mutant AML cells might exhibit a similar response to FLT3 TKIs. The expression of Bcl6 messenger RNA (mRNA) was thus measured by quantitative polymerase chain reaction (qPCR) following treatment of Molm14 cells with sorafenib (Figure 3A). A significant fivefold increase in Bcl6 expression was observed upon treatment with sorafenib, with mRNA levels peaking at 12 hours. This same pattern was observed by western blotting of cellular proteins, with the peak in Bcl6 protein level overexpression occurring by 24 hours of sorafenib exposure (Figure 3B-C). Similar results were observed upon treatment with the FLT3 TKIs AC220 and TTT-3002 (Figure 3C). Cotreatment with ATRA led to a reduction in Bcl6 expression back toward baseline levels (Figure 3A-C). In contrast, expression levels of Mcl-1, an antiapoptotic protein that has been previously shown to be modulated downstream of FLT3 inhibition,37 was decreased in response to both sorafenib and combination therapy both at the mRNA and protein level (Figure 3D-E). Analysis of additional Bcl family members (Bcl2, Bcl-xL) demonstrated that neither were induced by treatment with FLT3 TKIs, and thus do not appear to be involved in the partial resistance overcome by ATRA (supplemental Figure 4). Thus, FLT3 TKIs appear to increase Bcl6 expression in FLT3/ITD cells, and this upregulation of Bcl6 is reduced by cotreatment with ATRA.
ATRA abrogates sorafenib-mediated upregulation of Bcl6 via reduced STAT3 signaling. (A) Molm14 cells were treated with sorafenib (Sor, 20 nM) and/or ATRA (100 nM) for 6 to 48 hours, and mRNA expression of Bcl6 was measured in triplicate by qPCR relative to TATA-binding protein (TBP). Error bars indicate average fold change vs DMSO ± SD (**P < .01). Data are representative of 3 independent experiments. (B) Expression of phospho-FLT3 (pFLT3), total FLT3, Bcl6, and β-tubulin in Molm14 cells following 24-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM), representative of 4 independent experiments. (C) Bcl6 expression in Molm14 cells by western blotting following treatment with sorafenib (20 nM), AC220 (10 nM), or TTT-3002 (5 nM) alone or in combination with ATRA (100 nM) for 24 hours as in panel B, represented as average Bcl6 expression relative to β-tubulin vs DMSO for at least 3 independent experiments ± SD. (D) Molm14 cells were treated with sorafenib (20 nM) and/or ATRA (100 nM) for 12 or 24 hours, and mRNA expression of Mcl-1 was measured in triplicate by qPCR relative to TBP. Error bars indicate average fold change vs DMSO ± SD. Data are representative of 3 independent experiments. (E) Expression of Mcl-1 and β-tubulin in Molm14 cells following 24-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM), with fraction of Mcl-1/β-tubulin relative to DMSO control indicated below each blot. (F) Expression of phospho-STAT5 (pSTAT5), total STAT5, phospho-STAT3 (pSTAT3), and total STAT3 in Molm14 cells following 48-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM), with fraction of phospho-protein/total protein relative to DMSO indicated below each blot. Data are representative of 3 independent experiments. (G) Surface expression of CD126 (interleukin-6 receptor) in leukemia cell lines treated with sorafenib (20 nM) and/or ATRA (100 nM) for 48 hours, represented as relative CD126 mean fluorescence intensity (MFI) vs DMSO control. Error bars represent average of 3 independent experiments ± SD. (H) Expression of Bcl6 in Molm14 and THP-1 cells expressing NS shRNA or Bcl6-targeted (Bcl6_1, Bcl6_2, or Bcl6_3) shRNAs. Ratio of Bcl6 to β-tubulin relative to NS shRNA control is indicated below the blot. Data are representative of 3 independent experiments. (I) Annexin V binding in Molm14 and THP-1 cells with NS shRNA or Bcl6-targeted (Bcl6_1, Bcl6_2, Bcl6_3) shRNAs following 48-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM). Error bars represent average of 3 independent experiments ± SD (**P < .01).
ATRA abrogates sorafenib-mediated upregulation of Bcl6 via reduced STAT3 signaling. (A) Molm14 cells were treated with sorafenib (Sor, 20 nM) and/or ATRA (100 nM) for 6 to 48 hours, and mRNA expression of Bcl6 was measured in triplicate by qPCR relative to TATA-binding protein (TBP). Error bars indicate average fold change vs DMSO ± SD (**P < .01). Data are representative of 3 independent experiments. (B) Expression of phospho-FLT3 (pFLT3), total FLT3, Bcl6, and β-tubulin in Molm14 cells following 24-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM), representative of 4 independent experiments. (C) Bcl6 expression in Molm14 cells by western blotting following treatment with sorafenib (20 nM), AC220 (10 nM), or TTT-3002 (5 nM) alone or in combination with ATRA (100 nM) for 24 hours as in panel B, represented as average Bcl6 expression relative to β-tubulin vs DMSO for at least 3 independent experiments ± SD. (D) Molm14 cells were treated with sorafenib (20 nM) and/or ATRA (100 nM) for 12 or 24 hours, and mRNA expression of Mcl-1 was measured in triplicate by qPCR relative to TBP. Error bars indicate average fold change vs DMSO ± SD. Data are representative of 3 independent experiments. (E) Expression of Mcl-1 and β-tubulin in Molm14 cells following 24-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM), with fraction of Mcl-1/β-tubulin relative to DMSO control indicated below each blot. (F) Expression of phospho-STAT5 (pSTAT5), total STAT5, phospho-STAT3 (pSTAT3), and total STAT3 in Molm14 cells following 48-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM), with fraction of phospho-protein/total protein relative to DMSO indicated below each blot. Data are representative of 3 independent experiments. (G) Surface expression of CD126 (interleukin-6 receptor) in leukemia cell lines treated with sorafenib (20 nM) and/or ATRA (100 nM) for 48 hours, represented as relative CD126 mean fluorescence intensity (MFI) vs DMSO control. Error bars represent average of 3 independent experiments ± SD. (H) Expression of Bcl6 in Molm14 and THP-1 cells expressing NS shRNA or Bcl6-targeted (Bcl6_1, Bcl6_2, or Bcl6_3) shRNAs. Ratio of Bcl6 to β-tubulin relative to NS shRNA control is indicated below the blot. Data are representative of 3 independent experiments. (I) Annexin V binding in Molm14 and THP-1 cells with NS shRNA or Bcl6-targeted (Bcl6_1, Bcl6_2, Bcl6_3) shRNAs following 48-hour treatment with sorafenib (20 nM) and/or ATRA (100 nM). Error bars represent average of 3 independent experiments ± SD (**P < .01).
Bcl6 is known to contain binding sites for both STAT5 and STAT3 in its promoter region, with phospho-STAT5 downregulating Bcl6 transcription and phospho-STAT3 upregulating expression.38 Phosphorylation of STAT5, a key downstream signaling target of FLT3/ITD, is strongly inhibited by treatment with FLT3 TKIs such as sorafenib (Figure 3F). This would have the effect of relieving repression by STAT5 and leading to the observed increased Bcl6 expression. STAT3 is activated by the interleukin-6 receptor (CD126), among others, and this signaling pathway has been shown to play a role in Bcl6 regulation in the BM microenvironment.39 ATRA has been shown to downregulate surface expression of CD126.40 To investigate whether this might be the mechanism of suppressing Bcl6 expression in the setting of STAT5 inhibition by FLT3 TKIs, CD126 surface expression was measured by flow cytometry in leukemia cell lines following treatment with sorafenib, ATRA, or the combination (Figure 3G). Indeed, CD126 expression was decreased in cells treated with ATRA or sorafenib plus ATRA (and to a lesser extent sorafenib alone in MV4-11 cells), thus confirming the observation in the context of leukemia cell lines. As a consequence of the reduced CD126 expression, STAT3 phosphorylation was reduced in Molm14 cells treated with ATRA, correlating with the observed decrease in Bcl6 transcription (Figure 3F). Therefore, interference with STAT3 and STAT5 signaling may play a role in the synergy observed for the combination treatment of FLT3 TKIs and ATRA in FLT3/ITD+ AML.
Knockdown of Bcl6 diminishes the synergism of FLT3 TKI plus ATRA combination treatment of FLT3/ITD+ cells
To further explore the role of Bcl6 modulation in the synergistic effects observed with combined sorafenib and ATRA treatment, Molm14 and THP-1 cells were engineered to express nonsilencing (NS) or 1 of 3 Bcl6-targeted (Bcl6_1, Bcl6_2, and Bcl6_3) shRNAs under control of a doxycycline-inducible system. Following induction of shRNA expression, there was a significant decrease in basal Bcl6 protein levels in Molm14 and THP-1 cells compared with NS shRNA induction (Figure 3H). Upregulation of Bcl6 in Molm14 cells by sorafenib is also greatly reduced by knockdown with Bcl6-targeted shRNAs (data not shown). Consequently, upon treatment with sorafenib, there is an increase in Annexin V+ Molm14 cells compared with NS shRNA-expressing cells treated with the same dose of sorafenib (Figure 3I), whereas the control THP-1 cell line does not demonstrate increased Annexin V binding upon sorafenib treatment in the presence of Bcl6-targeted shRNA induction (Figure 3I). When treated with sorafenib and ATRA in combination, the NS shRNA-expressing Molm14 cells show the same extent of increased apoptosis as the Bcl6-targeted shRNA-expressing cells, indicating that ATRA is working at least in part through repression of Bcl6 (Figure 3I). Furthermore, treatment of Molm14 cells with 79-6, a Bcl6 inhibitor, in combination with sorafenib mimics the effects of ATRA in combination with sorafenib, with similar CI values observed by both MTT and Annexin V assays (supplemental Figure 5A-B).41 In contrast, overexpression of Bcl6 by retroviral transduction protects Molm14 cells from sorafenib and sorafenib plus ATRA-induced cell death (supplemental Figure 6A-B). Therefore, Bcl6 repression by ATRA is at least partly responsible for the synergistic effect observed for the combination treatment of sorafenib and ATRA in FLT3/ITD+ leukemia cell lines.
ATRA plus FLT3 TKI treatment reduces clonogenicity of FLT3/ITD+ cells
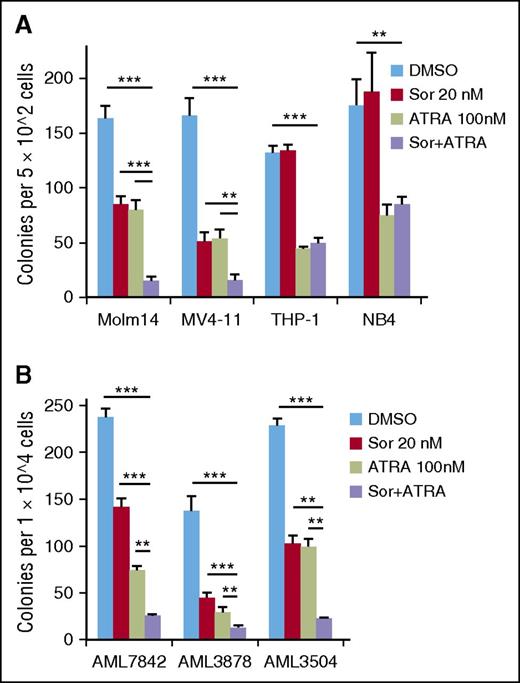
The effects of differentiating agents like ATRA on leukemic cells are difficult to measure by short-term proliferative or apoptosis assays, but these drugs can decrease the long-term proliferative potential that is characteristic of leukemia stem/progenitor cells. Therefore, CFU assays were performed on leukemia cell lines treated with sorafenib (20 nM) and/or ATRA (100 nM, Figure 4A). Although treatment with sorafenib or ATRA alone led to a 50% to 70% reduction in Molm14 and MV4-11 colonies, clonogenicity was strikingly decreased upon combination treatment, with >90% reduction in colonies from each of these FLT3/ITD+ cell lines (Figure 4A). In contrast, only ATRA significantly reduced clonogenicity of the FLT3/WT cell lines THP-1 and NB4, with no further reduction observed with combination treatment (Figure 4A). Similar results were obtained from FLT3/ITD+ patient blast samples that were capable of forming colonies on methylcellulose (50% of AML samples tested did not form colonies in this assay). Treatment with sorafenib plus ATRA resulted in 90% reduction of CFUs in each patient sample, whereas sorafenib or ATRA only reduced CFU counts by 40% to 67% and 57% to 79%, respectively (Figure 4B). In contrast, no drug effects were observed on normal hematopoietic stem/progenitor cell CFUs when treated with the same concentrations (supplemental Figure 7).
ATRA plus sorafenib reduces long-term proliferative potential of FLT3/ITD+ cells. (A) CFU counts after 7 to 10 days’ plating of 5 × 102 Molm14, MV4-11, THP-1, or NB4 cells treated in triplicate with sorafenib (Sor, 20 nM) and/or ATRA (100 nM) for 48 hours. (B) CFU counts after 7 days’ plating of 1 × 104 FLT3/ITD primary patient cells treated with sorafenib (20 nM) and/or ATRA (100 nM). Data indicate average colony number ± SD, and are representative of 3 independent experiments (**P < .01, ***P < .001).
ATRA plus sorafenib reduces long-term proliferative potential of FLT3/ITD+ cells. (A) CFU counts after 7 to 10 days’ plating of 5 × 102 Molm14, MV4-11, THP-1, or NB4 cells treated in triplicate with sorafenib (Sor, 20 nM) and/or ATRA (100 nM) for 48 hours. (B) CFU counts after 7 days’ plating of 1 × 104 FLT3/ITD primary patient cells treated with sorafenib (20 nM) and/or ATRA (100 nM). Data indicate average colony number ± SD, and are representative of 3 independent experiments (**P < .01, ***P < .001).
Sorafenib plus ATRA is efficacious in mouse xenograft models of FLT3/ITD AML
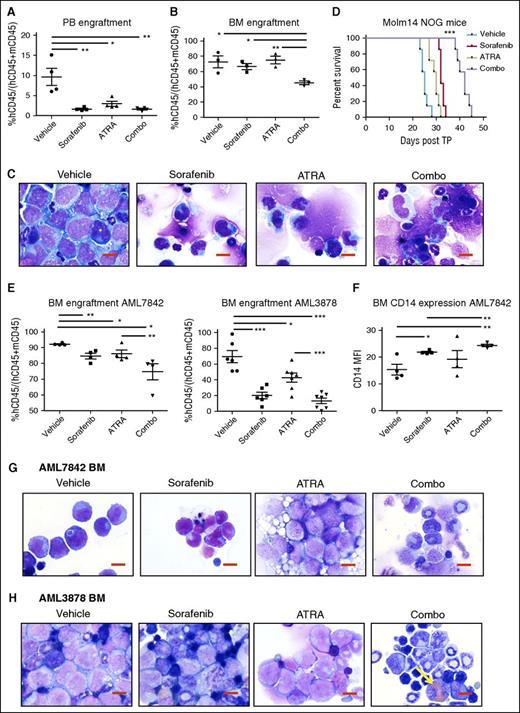
We next explored the efficacy of FLT3 TKI plus ATRA treatment in mouse xenograft models of AML. In vivo dosing of sorafenib (8 mg/kg daily), ATRA (5 mg/kg daily), and sorafenib plus ATRA in combination is well tolerated in NOG mice (supplemental Figure 8). Following transplantation of Molm14 cells, significant reductions were noted in the fraction of leukemic cells (hCD45+) in the peripheral blood (PB) of each drug-treated cohort (Figure 5A). However, only the combination treatment of ATRA plus sorafenib resulted in a reduction in leukemic cells in the BM (Figure 5B). BM smears were also examined for evidence of differentiation (Figure 5C). Vehicle-treated mice contained sheets of leukemic blasts in the BM, whereas sorafenib- and ATRA-treated mice displayed evidence of monocytic differentiation of Molm14 cells as well as normal background trilineage hematopoiesis. Combination-treated mice had even greater indications of differentiation and reduction of Molm14 cells by morphology, with increased cytoplasmic-to-nuclear ratios and nuclear folding of Molm14 cells, and a reduced fraction of leukemic cells overall in the BM. After 3 weeks, dosing was ceased and the remaining mice (n = 7 per cohort) were monitored for survival. Vehicle-treated mice succumbed to disease at 25 days posttransplant, ATRA- and sorafenib-treated mice survived an average of 30 and 32 days, respectively, and mice treated with sorafenib plus ATRA had significantly extended survival to a median of 42 days posttransplant (***P < .001, Figure 5D).
ATRA and sorafenib improve disease progression of AML and survival in mouse xenograft models. (A-E) NOG mice received 1 × 105 Molm14 cells via tail vain injection on day 0, and randomly assigned cohorts of mice (n = 10) were administered the following compounds on day 3 to day 24 posttransplant: sorafenib (8 mg/kg daily), ATRA (5 mg/kg daily), sorafenib + ATRA (Combo, 8 and 5 mg/kg, respectively) or vehicle, all via oral gavage. On day 24, n = 3 mice were sacrificed at random from each cohort and analyzed for disease progression. Remaining mice (n = 7 per cohort) were monitored for survival. (A) PB engraftment on day 24, represented by percentage human CD45+ (hCD45+) cells out of total of mouse CD45+ (mCD45+) and hCD45+ cells. Data represent average of n = 3 mice per cohort ± SD (*P < .05, **P < .01). (B) BM engraftment on day 24, represented as in panel A (*P < .05, **P < .01). (C) BM cellular morphology on day 24 by Wright-Giemsa staining at ×100 magnification. Reduction in fraction of leukemic cells and increased evidence of atypical monocytic differentiation is apparent in BM cells of combo-treated mice. Red scale bar represents 10 µm. Data are representative of n = 3 mice per cohort. (D) Kaplan-Meier survival of mouse cohorts (n = 7 each), indicating median survival of vehicle (25 days), ATRA (30 days), sorafenib (32 days), and combo (42 days) treated mice (***P < .001). (E-H) NOG mice received 1 × 106 AML7842 or AML3878 primary cells via tail vain injection, and randomly assigned cohorts of mice (n = 4-7) were treated as described above for 3 weeks. At end of treatment, mice were sacrificed and analyzed for disease progression. (E) BM engraftment at end of treatment, represented as in panel A. Data represent average of n = 4-7 mice per cohort ± SD (*P < .05, **P < .01, ***P < .001). (F) BM CD14 expression in AML7842 recipient mice, represented by MFI of human CD14 in hCD45+ cells. Data represent average of n = 4 mice per cohort ± SD (*P < .05, **P < .01). (G-H) BM cellular morphology at end of treatment of (G) AML7842 and (H) AML3878 recipient mice by Wright-Giemsa staining at ×100 magnification. Reduction in fraction of leukemic cells and increased evidence of granulocytic and monocytic differentiation is apparent in BM cells of combo-treated mice. Yellow arrow indicates cell with iron particle deposition. Red scale bar represents 10 µm. Data are representative of n = 4-7 mice per cohort.
ATRA and sorafenib improve disease progression of AML and survival in mouse xenograft models. (A-E) NOG mice received 1 × 105 Molm14 cells via tail vain injection on day 0, and randomly assigned cohorts of mice (n = 10) were administered the following compounds on day 3 to day 24 posttransplant: sorafenib (8 mg/kg daily), ATRA (5 mg/kg daily), sorafenib + ATRA (Combo, 8 and 5 mg/kg, respectively) or vehicle, all via oral gavage. On day 24, n = 3 mice were sacrificed at random from each cohort and analyzed for disease progression. Remaining mice (n = 7 per cohort) were monitored for survival. (A) PB engraftment on day 24, represented by percentage human CD45+ (hCD45+) cells out of total of mouse CD45+ (mCD45+) and hCD45+ cells. Data represent average of n = 3 mice per cohort ± SD (*P < .05, **P < .01). (B) BM engraftment on day 24, represented as in panel A (*P < .05, **P < .01). (C) BM cellular morphology on day 24 by Wright-Giemsa staining at ×100 magnification. Reduction in fraction of leukemic cells and increased evidence of atypical monocytic differentiation is apparent in BM cells of combo-treated mice. Red scale bar represents 10 µm. Data are representative of n = 3 mice per cohort. (D) Kaplan-Meier survival of mouse cohorts (n = 7 each), indicating median survival of vehicle (25 days), ATRA (30 days), sorafenib (32 days), and combo (42 days) treated mice (***P < .001). (E-H) NOG mice received 1 × 106 AML7842 or AML3878 primary cells via tail vain injection, and randomly assigned cohorts of mice (n = 4-7) were treated as described above for 3 weeks. At end of treatment, mice were sacrificed and analyzed for disease progression. (E) BM engraftment at end of treatment, represented as in panel A. Data represent average of n = 4-7 mice per cohort ± SD (*P < .05, **P < .01, ***P < .001). (F) BM CD14 expression in AML7842 recipient mice, represented by MFI of human CD14 in hCD45+ cells. Data represent average of n = 4 mice per cohort ± SD (*P < .05, **P < .01). (G-H) BM cellular morphology at end of treatment of (G) AML7842 and (H) AML3878 recipient mice by Wright-Giemsa staining at ×100 magnification. Reduction in fraction of leukemic cells and increased evidence of granulocytic and monocytic differentiation is apparent in BM cells of combo-treated mice. Yellow arrow indicates cell with iron particle deposition. Red scale bar represents 10 µm. Data are representative of n = 4-7 mice per cohort.
Engraftment of primary human leukemia cells from 2 FLT3/ITD+ patients (AML7842, AML3878) was detectable in NOG mice at 4 and 8 weeks posttransplant, respectively. Mice were then randomly divided into 4 cohorts and treated as described previously for the Molm14 transplanted mice. After 3 weeks of daily dosing, significant reductions in BM engraftment were observed in drug-treated mice (Figure 5E), with the greatest decreases in hCD45+ cells observed in the BM of combination-treated mice (P < .05 and P < .0001 for AML7842 and AML3878, respectively). Evidence of myeloid differentiation by increased expression of CD14 was also observed in the BM of drug-treated AML7842 recipient mice (Figure 5F). Morphologic analysis of BM smears of AML7842 recipient mice revealed increased monocytic differentiation in sorafenib- and combination-treated mice, as evidenced by nuclear folding and increased cytoplasmic-to-nuclear ratios, and granules were clearly present in the cells of mice treated with ATRA or the combination (Figure 5G). AML3878 recipient mice had evidence of increased myeloid differentiation in the BM of combination-treated mice, as well as significant deposition of iron particles, indicating leukemia cell killing (Figure 5H).
Sorafenib and ATRA treatment targets leukemia stem cells
To further explore the possible targeting of LSCs by the combination of sorafenib and ATRA in primary murine leukemic cells as opposed to cell lines, we used a genetic mouse model of FLT3/ITD+ AML. Knock-in of FLT3/ITD in cooperation with transgenic expression of the Nup98-HoxD13 fusion (NHD13) generates a fully transformed, spontaneous AML in 4 to 5 months with 100% penetrance in the double-positive progeny of the crosses (NHD13;FLT3/ITD).30 Similar to our results with leukemia cell lines and patient samples, ex vivo treatment of BM from these leukemic mice with sorafenib plus ATRA results in decreased clonogenicity compared with dimethyl sulfoxide (DMSO)- and monotherapy-treated cells by CFU counts (Figure 6A). The NHD13;FLT3/ITD mouse model has a well-defined immunophenotype for the LSCs, which is present at a frequency of ∼1:20 cells in the multipotent progenitor (MPP; Lin−Sca-1hicKIThiCD34+CD135+) cell population from the leukemic mice.30 We used MPP cells isolated from a leukemic donor mouse (CD45.2+) and treated them ex vivo with ATRA or DMSO for 48 hours. Equal numbers of viable cells were then transplanted into recipients (CD45.1+), and mice were further randomly divided into vehicle (Veh)-treated or sorafenib (Sor)-treated cohorts, resulting in 4 distinct treatment groups: DMSO/Veh, DMSO/Sor, ATRA/Veh, and ATRA/Sor. Following a 7-day engraftment period, mice were treated with vehicle or sorafenib for 2 weeks, and then monitored for survival. At 8 weeks (day 58) posttransplant, PB was analyzed for engraftment of CD45.2+ cells by flow cytometry (see Figure 6B for schematic). A significant decrease in CD45.2+ donor cells was observed in mice treated with sorafenib, and an even greater decrease was observed in mice treated with the combination of sorafenib plus ATRA (Figure 6C). This correlated with an increase in median survival in all 3 drug treatment groups (Figure 6D).
ATRA plus sorafenib targets leukemia stem cells in a genetically engineered mouse model of AML. (A) CFU counts at day 7 of 5 × 103 NHD13;FLT3/ITD leukemic mouse BM cells treated with sorafenib (20 nM) and/or ATRA (100 nM). Data indicate average colony number ± SD, and are representative of 3 independent experiments (**P < .01, ***P < .001). (B-D) MPP cells were isolated from a CD45.2+ NHD13;FLT3/ITD leukemic donor mouse and treated with DMSO or ATRA (10 nM) ex vivo for 48 hours. On day 2, 1 × 104 viable donor cells along with 5 × 105 CD45.1+ helper cells were transplanted into sublethally irradiated CD45.1+ recipients (n = 20) and randomly divided into 4 cohorts: DMSO/Veh, DMSO/Sor, ATRA/Veh, ATRA/Sor. Starting on day 9, mice received vehicle (Veh) or sorafenib (Sor) for 2 weeks. (B) Mouse xenograft schematic. (C) PB engraftment on day 58 posttransplant, represented by percentage CD45.2+ cells of a total of CD45.1+ and CD45.2+ cells. Data represent average of n = 5 mice per cohort ± SD (*P < .05, **P < .01, ***P < .001). (D) Kaplan-Meier survival of mouse cohorts (n = 5 each), indicating median survival of DMSO/Veh (69 days), DMSO/Sor (109 days), ATRA/Veh (80 days), and ATRA/Sor (127) days (**P < .01, relative to vehicle). Treatment period is indicated by black arrows. (E) Mice received 5 × 105 whole BM cells from a NHD13;FLT3/ITD leukemic donor along with 5 × 105 CD45.1+ helper cells and were treated in vivo with sorafenib (5 mg/kg daily), ATRA (5 mg/kg daily), sorafenib + ATRA (Sor+ATRA), or vehicle, all via oral gavage, for 2 weeks. Kaplan-Meier survival of mouse cohorts (n = 6 each), indicating median survival of vehicle (26 days), ATRA (25 days), sorafenib (34 days), and Sor+ATRA (55.5) days (**P < .01, ***P < .001, relative to vehicle). Treatment period is indicated by black arrows. (F) Mouse secondary transplant schematic. Whole BM cells were isolated from a CD45.2+ NHD13;FLT3/ITD leukemic donor mouse and 5 × 105 donor cells, along with 5 × 105 CD45.1+ helper cells, were transplanted into sublethally irradiated CD45.1+ recipients. Starting on day 7, mice received vehicle, sorafenib, ATRA, or Sor+ATRA (n = 3 per cohort) for 2 weeks. PB and BM were harvested on day 21 posttransplant, and pooled BM was transplanted into healthy secondary recipients and mice were monitored for PB engraftment and survival. (G) PB engraftment on day 28 and (H) day 84 postsecondary transplant, represented by the percentage of CD45.2+ cells of a total of CD45.1+ and CD45.2+ cells. Data represent average of n = 3 mice per cohort ± SD (**P < .01, ***P < .001). (I) Kaplan-Meier survival of mouse cohorts (n = 5 each), indicating median survival of vehicle (43 days), ATRA (96 days), sorafenib (>120 days), and Sor+ATRA (>120 days) treated mouse BM recipient mice (**P < .01 for vehicle to ATRA, vehicle to sorafenib, and vehicle to Sor+ATRA comparisons, *P < .05 for ATRA to Sor+ATRA comparison). (J) Limiting dilution transplantation analysis of pooled whole BM from mice treated as in panels E and F. Detection of >1% hCD45+ of a total of CD45.1+ and CD45.2+ cells in the PB was used as a marker for successful engraftment of leukemia. TP, transplant; WBM, whole bone marrow.
ATRA plus sorafenib targets leukemia stem cells in a genetically engineered mouse model of AML. (A) CFU counts at day 7 of 5 × 103 NHD13;FLT3/ITD leukemic mouse BM cells treated with sorafenib (20 nM) and/or ATRA (100 nM). Data indicate average colony number ± SD, and are representative of 3 independent experiments (**P < .01, ***P < .001). (B-D) MPP cells were isolated from a CD45.2+ NHD13;FLT3/ITD leukemic donor mouse and treated with DMSO or ATRA (10 nM) ex vivo for 48 hours. On day 2, 1 × 104 viable donor cells along with 5 × 105 CD45.1+ helper cells were transplanted into sublethally irradiated CD45.1+ recipients (n = 20) and randomly divided into 4 cohorts: DMSO/Veh, DMSO/Sor, ATRA/Veh, ATRA/Sor. Starting on day 9, mice received vehicle (Veh) or sorafenib (Sor) for 2 weeks. (B) Mouse xenograft schematic. (C) PB engraftment on day 58 posttransplant, represented by percentage CD45.2+ cells of a total of CD45.1+ and CD45.2+ cells. Data represent average of n = 5 mice per cohort ± SD (*P < .05, **P < .01, ***P < .001). (D) Kaplan-Meier survival of mouse cohorts (n = 5 each), indicating median survival of DMSO/Veh (69 days), DMSO/Sor (109 days), ATRA/Veh (80 days), and ATRA/Sor (127) days (**P < .01, relative to vehicle). Treatment period is indicated by black arrows. (E) Mice received 5 × 105 whole BM cells from a NHD13;FLT3/ITD leukemic donor along with 5 × 105 CD45.1+ helper cells and were treated in vivo with sorafenib (5 mg/kg daily), ATRA (5 mg/kg daily), sorafenib + ATRA (Sor+ATRA), or vehicle, all via oral gavage, for 2 weeks. Kaplan-Meier survival of mouse cohorts (n = 6 each), indicating median survival of vehicle (26 days), ATRA (25 days), sorafenib (34 days), and Sor+ATRA (55.5) days (**P < .01, ***P < .001, relative to vehicle). Treatment period is indicated by black arrows. (F) Mouse secondary transplant schematic. Whole BM cells were isolated from a CD45.2+ NHD13;FLT3/ITD leukemic donor mouse and 5 × 105 donor cells, along with 5 × 105 CD45.1+ helper cells, were transplanted into sublethally irradiated CD45.1+ recipients. Starting on day 7, mice received vehicle, sorafenib, ATRA, or Sor+ATRA (n = 3 per cohort) for 2 weeks. PB and BM were harvested on day 21 posttransplant, and pooled BM was transplanted into healthy secondary recipients and mice were monitored for PB engraftment and survival. (G) PB engraftment on day 28 and (H) day 84 postsecondary transplant, represented by the percentage of CD45.2+ cells of a total of CD45.1+ and CD45.2+ cells. Data represent average of n = 3 mice per cohort ± SD (**P < .01, ***P < .001). (I) Kaplan-Meier survival of mouse cohorts (n = 5 each), indicating median survival of vehicle (43 days), ATRA (96 days), sorafenib (>120 days), and Sor+ATRA (>120 days) treated mouse BM recipient mice (**P < .01 for vehicle to ATRA, vehicle to sorafenib, and vehicle to Sor+ATRA comparisons, *P < .05 for ATRA to Sor+ATRA comparison). (J) Limiting dilution transplantation analysis of pooled whole BM from mice treated as in panels E and F. Detection of >1% hCD45+ of a total of CD45.1+ and CD45.2+ cells in the PB was used as a marker for successful engraftment of leukemia. TP, transplant; WBM, whole bone marrow.
Given these results, we next wanted to test the ability of the drug combination to eliminate LSCs fully in vivo. In vivo treatment of mice transplanted with NHD13;FLT3/ITD leukemic donor BM with vehicle, sorafenib, ATRA, and the combination for 2 weeks results in increased median survival in sorafenib- and combination-treatment groups (P < .01 and P < .001, relative to vehicle, Figure 6E). In a similar experiment (see Figure 6F for schematic), secondary transplantation of pooled BM harvested from each of the treatment cohorts into healthy recipient mice revealed significant reductions in LSCs of the combination-treated cohort as measured by engraftment of >1% CD45.2+ cells in the PB at 4 weeks posttransplant. Fractions of engrafted recipient mice were as follows: vehicle, 5 of 5 (100%), sorafenib, 3 of 5 (60%), ATRA, 5 of 5 (100%), combination 0 of 5 (0%). Furthermore, the level of PB engraftment was reduced in sorafenib, ATRA, and combination-treated mouse BM recipients compared with vehicle-treated BM recipients at day 28 postsecondary transplant (Figure 6G). PB engraftment was measured again in the remaining surviving mice on day 84 postsecondary transplant, and the combination-treated BM recipients still had no evidence of engraftment, whereas the CD45.2+ cells continued to expand in the PB of the sorafenib- and ATRA-treated BM recipients (Figure 6H). All of the mice transplanted with BM from the vehicle-treated or ATRA-treated cohort succumbed to leukemia (median survival of 43 and 96 days, respectively), and several from the sorafenib-treated cohort (median survival not reached, Figure 6I). However, all of the mice transplanted from the combination-treated cohort were still alive at day 155 posttransplant, indicating that the 2-week in vivo treatment of primary recipient mice appeared to have reduced the LSC population to levels below which it was able to engraft the secondary recipients. Finally, limiting dilution transplantation of pooled BM harvested from mice treated with vehicle, sorafenib, ATRA, or the combination revealed that the absolute frequency of LSCs in the BM was reduced from ∼1:80 in the vehicle-treated mice to <1:1 000 000 whole BM cells from combination-treated mice. Monotherapy with sorafenib or ATRA reduced LSC frequency to ∼1:37 848 and 1:1696 cells, respectively (Figure 6J; supplemental Figure 9A-B). Based on these studies, it seems probable that combining a FLT3 TKI with ATRA in human patients may reduce the rate of relapse by targeting not only the bulk leukemia cells but, more importantly, the LSC population.
Discussion
Although continued efforts are being made to successfully combine FLT3 TKIs with chemotherapy, we also now have a better understanding of the molecular alterations of certain pathways that frequently occur in AML. Therefore, the exploration of additional targeted therapeutic agents that may synergize with FLT3 inhibition could move clinical trials toward the goal of eliminating chemotherapy altogether. Aside from its use in APL, ATRA (in combination with chemotherapy) has demonstrated clinical activity in nonpromyelocytic patients with NPM1 mutations.24 Furthermore, the role of ATRA in the differentiation of HSCs makes this pathway a desirable candidate for pharmacologic manipulation, providing a means to target common pathways downstream of mutations contributing to a block in differentiation of LSCs.
The results of the present study demonstrate synergy for the combination of FLT3 TKIs plus ATRA in vitro and improved survival in vivo compared with either drug alone when used to treat both FLT3-mutant AML cell lines and primary leukemias. The studies reported here also explore the possible cellular and molecular mechanisms underlying the observed synergy between FLT3 TKIs and ATRA. There was evidence for improved targeting of LSCs given the eradication of transplantable NHD13;FLT3/ITD LSCs in mice treated with the combination of ATRA and sorafenib. Given the prodifferentiation activity of ATRA alone, one can postulate that ATRA pushes LSCs out of their quiescent state, thereby making them more susceptible to the cytotoxic activity of sorafenib in vivo. At the molecular level, upregulation of Bcl6 by FLT3 TKIs was shown to reduce the cytotoxic effect of these drugs, but this resistance can be reversed with the addition of ATRA in combination. STAT3 and STAT5 are implicated in this mechanism due to their inverse transcriptional regulation of Bcl6, and the fact that they lie downstream of ATRA- and sorafenib-mediated signaling modulators, respectively. However, it is likely that this finding does not fully account for the synergistic activity of these drugs. Therefore, further elucidation of the crosstalk of FLT3 and ATRA signaling pathways is warranted. Interestingly, the modulation of Bcl6 by FLT3 TKIs and ATRA demonstrated here is reminiscent of previous work in chronic myeloid leukemia and Ph+ acute lymphoblastic leukemia, whereby the combination of TKIs and Bcl6 inhibition effectively eradicated LSCs.35,36 If Bcl6 is downregulated in a similar manner upon treatment with ATRA, it provides further explanation as to how ATRA is able to reduce clonogenicity and LSCs in our cell line and mouse models of AML.
We believe these preclinical data are encouraging for the development of a clinical trial of ATRA plus a FLT3 TKI in relapsed/refractory or in upfront elderly FLT3-mutant AML patients. Such trials should be quite feasible as the individual agents are well tolerated and can be administered entirely as outpatient therapy. Indeed, a recent study reported 3 FLT3/ITD+/NPM1+ AML patients who achieved significant responses when treated with sorafenib and ATRA in combination.42
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health National Cancer Institute (CA90668, CA006973), the Rally Foundation (The Truth 365 grant), and the Giant Food Pediatric Cancer Research Fund. D.S. is also supported by the Kyle Haydock Professorship.
Authorship
Contribution: H.S.M. designed and performed experiments, analyzed data, and wrote the manuscript; S.M.G., C.M.S., A.S.D., and J.K.B. designed and performed experiments and analyzed data; L.L., B.N., and E.J. performed experiments; P.D.A. provided the mouse model; G.G. and R.J.J. designed experiments and provided patient samples; and D.S. designed experiments, analyzed data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald Small, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, CRB1, 1650 Orleans St, Baltimore, MD 21231; e-mail: donsmall@jhmi.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal