Key Points
BCR signals induce ABT-199 resistance in CLL cells by upregulating Mcl-1.
SYK inhibitors prevent BCR-mediated Mcl-1 induction more effectively than BTK or PI3Kδ inhibitors.
Abstract
The Bcl-2 antagonist ABT-199 (venetoclax) has demonstrated promising clinical activity in patients with chronic lymphocytic leukemia (CLL). ABT-199 is strongly cytotoxic against unstimulated peripheral blood CLL cells in vitro but is much less effective against CLL cells that have received survival signals from the microenvironment. In particular, stimulation of CLL cells with CD40L results in substantial resistance mediated by induction of the antiapoptotic Bcl-2 family proteins Bcl-xL and Bfl-1. In this study, we investigated whether resistance to ABT-199 can be conferred by B-cell receptor (BCR) stimulation, which is another important survival signal from the leukemic microenvironment. We show that sustained BCR stimulation results in significant ABT-199 resistance, which correlates with induction of the antiapoptotic protein Mcl-1 and less consistently with downregulation of proapoptotic Bmf, Hrk, and BimEL. A major role for Mcl-1 in conferring ABT-199 resistance is additionally supported by knockdown and enforced expression experiments with primary CLL cells. We further show that SYK, BTK, and phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitors significantly downregulate Mcl-1, but with different efficacy. Complete Mcl-1 downregulation was consistently achieved only with SYK inhibitors R406 and GS-9973 (entospletinib), whereas the BTK inhibitor ibrutinib and the PI3Kδ inhibitor idelalisib in more than half of the cases had only a partial effect. The greater ability of SYK inhibitors to downregulate Mcl-1 correlated with their greater capacity to block BCR-mediated inactivation of GSK-3, a major negative regulator of Mcl-1. The finding that BCR signaling inhibitors differ in their ability to target Mcl-1 is relevant for the design of clinical trials combining these agents with ABT-199.
Introduction
Inefficient apoptosis is a hallmark of chronic lymphocytic leukemia (CLL). Overexpression of the antiapoptotic protein Bcl-2 is considered primarily responsible for the increased apoptosis resistance and prolonged survival of CLL B cells.1,2 The reasons for Bcl-2 overexpression in CLL are still not completely understood, but in patients with del13q14 this is likely caused by deletion of microRNAs miR-15a and miR-16, which have been shown to downmodulate Bcl-2 expression.3 In addition, apoptosis resistance of CLL cells is further increased by various microenvironmental signals that the leukemic cells receive primarily in the tissues.4 These signals increase the expression of other antiapoptotic Bcl-2 family proteins, including Mcl-1, Bcl-xL, and Bfl-1/A1.5-7
Antiapoptotic Bcl-2 family proteins suppress apoptosis by 2 mechanisms. The first mechanism is by binding and preventing the oligomerization of the multidomain proapoptotic Bcl-2 family proteins Bax and Bak, which initiate apoptosis by forming pores in the mitochondrial outer membrane that result in cytochrome c release and subsequent caspase activation. The second mechanism is by binding and sequestering the propapoptotic Bcl-2 BH3-only proteins Bim and Bid, which induce the conformational changes in Bax and Bak that are required for their activation.
Drugs that mimic proapoptotic BH3-only proteins have recently been developed and have demonstrated considerable activity against CLL cells both in preclinical models and clinical trials (reviewed in Kang and Reynolds8 and Balakrishnan and Gandhi9 ). These so-called BH3 mimetics disrupt the interactions between antiapoptotic and proapoptotic Bcl-2 family proteins, resulting in release of Bim and subsequent Bax and Bak oligomerization.10,11 Among the different BH3 mimetics that have been tested in CLL, particularly promising activity has been observed with ABT-737 and ABT-199, which are 2 related compounds that differ in their selectivity for antiapoptotic Bcl-2 family proteins. ABT-737 and its orally active analog ABT-263 bind to Bcl-2, Bcl-xL, and Bcl-W, whereas they do not bind to the 2 other antiapoptotic Bcl-2 family members Mcl-1 and Bfl-1. ABT-263 demonstrated considerable activity in a phase 1 study of patients with relapsed/refractory CLL but induced frequent dose-dependent thrombocytopenia because of inhibition of Bcl-xL, a key survival protein in platelets.12 To circumvent this undesirable toxicity, ABT-263 was reengineered into ABT-199, which binds selectively to Bcl-2.13 ABT-199 was well tolerated and induced a high response rate in a phase 1 study of relapsed/refractory CLL/small lymphocytic lymphoma, suggesting that it could represent a novel effective therapy in CLL.14
Interestingly, despite the considerable toxicity of ABT-263 and ABT-199 against CLL cells in vitro, relatively few complete responses were observed in these trials, suggesting that in vivo mechanisms must exist that can increase the resistance of the leukemic cells to these drugs. One such mechanism could involve stimulation of CLL cells by CD40L-expressing T cells, which are known to interact with the leukemic cells in the lymph node microenvironment and to provide survival signals through the CD40L/CD40 interaction. Such a mechanism is further supported by findings from recent in vitro studies, demonstrating that stimulation of CLL cells with CD40L confers almost full protection to ABT-263 and ABT-199, mediated by induction of the antiapoptotic proteins Bfl-1 and Bcl-xL.15,16
In the present study, we investigated whether resistance to ABT-199 can be conferred by engagement of the B-cell receptor (BCR), which is another important stimulus that CLL cells receive primarily in the lymph nodes.17 Previous studies by our group have shown that sustained engagement of the BCR induces Mcl-1,6 and high levels of Mcl-1 were shown to inversely correlate with treatment response in the phase 1 study of ABT-263 in CLL.12 In addition, high levels of Mcl-1 have been shown to protect other hematological malignancies and certain solid tumors from ABT-199,18-20 suggesting that Mcl-1 could also confer ABT-199 resistance to CLL cells. To further explore this possibility, we evaluated whether BCR-induced Mcl-1 overexpression can protect CLL cells from ABT-199 and whether this protection can be overcome by available BCR signaling inhibitors.
Materials and methods
CLL samples and culture conditions
Blood samples were collected from patients who satisfied standard morphologic and immunophenotypic criteria for CLL. Patients were untreated or had not received treatment for at least 6 months prior to the study. Informed consent was obtained from all patients according to the Declaration of Helsinki, and approval for the study was obtained from the institutional human research committee at the Catholic University Hospital A. Gemelli. Clinical and laboratory features of the studied patients are provided in supplemental Table 1 (available on the Blood Web site).
Mononuclear cells were isolated from peripheral blood samples by Ficoll gradient centrifugation. The proportion of CD5+CD19+ CLL cells was >80% in all analyzed cases. CLL cells were cultured at a cell density of 1 × 107/mL in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate (Invitrogen). Goat F(ab′)2 anti–human immunoglobulin M (IgM) 10 μg/mL (Southern Biotechnology Associates) or Dynabeads M-450 Epoxy 2 × 107/mL (Invitrogen Dynal) coated with 20 μg/mL goat anti–human IgM (Southern Biotechnology Associates) were used for BCR cross-linking. The Bcl-2 inhibitor ABT-199, the SYK inhibitors R406 and GS-9973, the BTK inhibitor ibrutinib, and the phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitor idelalisib (all from Selleckchem), as well as the pan–protein kinase C (PKC) inhibitor sotrastaurin (AXON Medchem), were used as indicated in the figures or figure legends.
Immunoblotting analysis
Cells were washed in ice-cold phosphate-buffered saline, pelleted, and lysed in radioimmunoprecipitation assay buffer lysis buffer (10 mM tris(hydroxymethyl)aminomethane-HCl, pH 7.4, 5 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.1% sodium deoxycholate) containing protease and phosphatase inhibitors (Sigma-Aldrich). The protein concentration of each cell lysate was determined with the RC DC Protein Assay (Bio-Rad). The protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred on Immobilon-P polyvinylidene difluoride membranes (Millipore). Membranes were blotted at 4°C in the presence of 5% nonfat dry milk with the following antibodies: PARP (cat. 9542), Bim (cat. 2819), Bcl-2 (cat. 2876), phospho-AKTS473 (cat. 9271), AKT (cat. 9272), phospho-ERKT202/Y204 (cat. 9101), extracellular signal-regulated kinase (ERK; cat. 9102), phospho-GSK-3α/βS21/9 (cat. 9331), phospho-FoxO1T24/FoxO3aT32 (cat. 9464), β-actin (cat. 3700), rabbit IgG–horseradish peroxidase linked, and mouse IgG–horseradish peroxidase linked (all from Cell Signaling Technology), Mcl-1 (cat. sc-819), Bcl-xL (cat. sc-1041), and β-catenin (cat. sc-7199) (Santa Cruz Biotechnology) or phospho-PLCγ2Y759 (cat. 558490) (BD Biosciences). Immunodetection and quantification were done on a Gel Logic 2200 Imaging System (Eastman Kodak), using ECL Plus enhanced-chemiluminescence detection reagents (GE Healthcare).
Real-time RT/PCR analysis (quantitative RT-PCR)
Total cellular RNA was isolated from CLL B cells using the Trizol reagent (Life Technologies). Complementary DNAs were generated from 250 to 500 ng of total RNA using random hexamers, MuLV Reverse Transcriptase, and RNAse inhibitor, according to the manufacturer’s instruction (Life Technologies). Quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad) using TaqMan gene expression assays for Hrk, Bmf, Bfl-1, and actin, according to the manufacturer’s recommendations (Life Technologies). Samples were run in triplicate, and relative quantification was performed with the comparative Ct method (2−ΔCt, where ΔCt = Ct Hrk or Ct Bmf or Ct Bfl-1 − Ct actin).
Mcl-1 knockdown and overexpression experiments
The Mcl-1 knockdown experiments were performed using a dicer-substrate short interfering (siRNA) with the following sequence: sense strand 5′ CGAAGGAAGUAUCGAAUUUACAUTA 3′, antisense strand 5′ UAAUGUAAAUUCGAUACUUCCUUCGUU 3′ (IDT). Capped and poly(A) tailed Mcl-1 and control messenger RNA (mRNA) were prepared from an Mcl-1 pCDNA3 expression vector (kindly provided by Hidenori Ichijo)21 and empty pCDNA3, respectively, using the mMESSAGE mMACHINE T7 Ultra Kit and purified with the MEGAclear kit (both from Ambion). Nucleofection of siRNAs or mRNAs in primary CLL B cells was performed with the Nucleofector system (Amaxa Biosystems) using Nucleofector Solution V and the U-013 program, as previously described.22
Apoptosis assay
The percentages of viable and apoptotic cells were determined by flow cytometric analysis of cells stained with propidium iodide (PI) and annexin A5–fluorescein isothiocyanate conjugate (Nexins Research).
Statistical analysis
Data are expressed as means ± standard deviation. The paired Student t test, Wilcoxon signed-rank test, and one-way repeated measures analysis of variance were used to evaluate the significance of differences in leukemic cell survival, as indicated in the figure legends. Wilcoxon signed-rank test was used to evaluate changes in Mcl-1 expression induced by the different BCR signaling inhibitors. The relationship between Mcl-1 expression and the rate of anti-IgM–induced protection from ABT-199–mediated apoptosis was evaluated through the Pearson rank correlation. All statistical analyses were performed using the SigmaStat Version 3.1 program (Systat Software). P values are indicated in the figures.
Results
BCR signals protect CLL cells from ABT-199 by upregulating Mcl-1
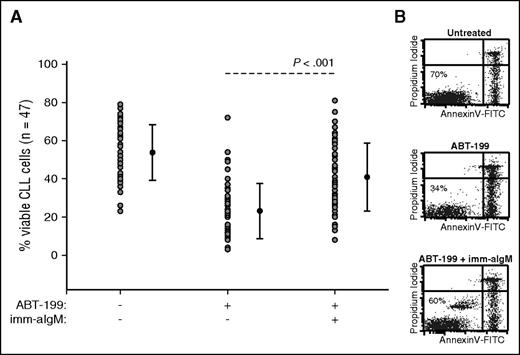
Previous studies by our group had shown that immobilized anti-IgM antibodies induce a sustained BCR signal that protects CLL cells from spontaneous and chemotherapy-induced apoptosis.6,22 To determine whether resistance to ABT-199 can also be induced by BCR signals, we investigated induction of apoptosis in CLL cells from 47 patients in the presence or absence of immobilized anti-IgM antibodies. ABT-199 was added 3 hours after immobilized anti-IgM at a concentration of 2 nM, which corresponds to the in vitro median lethal dose concentration of this drug13 (and data not shown). CLL cell viability was evaluated 24 hours later by annexin V/PI staining. As shown in Figure 1 and supplemental Table 1, anti-IgM stimulation significantly protected CLL cells from ABT-199–induced apoptosis. The level of protection appeared greater in samples with unmutated than mutated IGHV genes, but the difference was not statistically significant between the 2 groups (supplemental Figure 1).
BCR-stimulated CLL cells are resistant to ABT-199–mediated apoptosis. (A) CLL cells (n = 47) were cultured for 24 hours with or without ABT-199 or ABT-199 + immobilized anti-IgM (imm-aIgM). ABT-199 (2 nM) was added 3 hours after imm-aIgM. The percentage of viable cells was determined by annexin V/PI staining and flow cytometry. Significance of differences in leukemic cell viability was evaluated with the paired Student t test. (B) One representative experiment is shown. Viable cells bound with beads coated with imm-aIgM are seen as a separate annexin V–negative/PI-negative population.
BCR-stimulated CLL cells are resistant to ABT-199–mediated apoptosis. (A) CLL cells (n = 47) were cultured for 24 hours with or without ABT-199 or ABT-199 + immobilized anti-IgM (imm-aIgM). ABT-199 (2 nM) was added 3 hours after imm-aIgM. The percentage of viable cells was determined by annexin V/PI staining and flow cytometry. Significance of differences in leukemic cell viability was evaluated with the paired Student t test. (B) One representative experiment is shown. Viable cells bound with beads coated with imm-aIgM are seen as a separate annexin V–negative/PI-negative population.
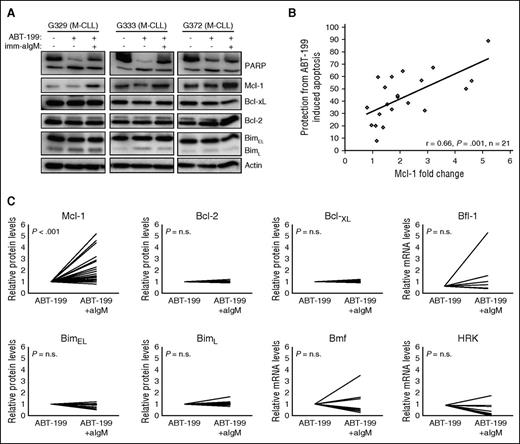
To characterize the mechanism of protection, we evaluated changes in the expression of several antiapoptotic and proapoptotic Bcl-2 family proteins that have been reported to play an important role in regulating CLL cell survival (Bcl-2, Mcl-1, Bcl-xL, Bfl-1, Bim, and Bmf)5-7,23 or that have been linked to BCR-mediated apoptosis in other B-cell malignancies (Hrk).24 As previously reported,6 stimulation with immobilized anti-IgM induced a considerable increase in Mcl-1 protein levels in most investigated samples, with a trend toward greater induction in samples with unmutated IGHV genes (Figure 2; supplemental Figure 2). Correlation analysis between the change in Mcl-1 expression and the relative increase in the percentage of viable cells showed that samples with a greater increase in Mcl-1 expression were more strongly protected, indicating that Mcl-1 is directly involved in mediating anti-IgM–induced ABT-199 resistance (Figure 2B). In contrast, no substantial changes were observed in the protein levels of Bcl-2, Bcl-xL, and BimL, whereas BimEL showed a substantial reduction (>25%) in 4 of the 11 investigated cases (Figure 2C). Because Bfl-1, Bmf, and Hrk could not be detected by immunoblotting analysis, we investigated their expression levels in unstimulated and anti-IgM–stimulated ABT-199–treated cells by real-time PCR. A >25% reduction in the levels of Bmf and Hrk was observed in 5 of the 8 investigated cases, whereas Bfl-1 showed a substantial increase (less than twofold) in 2 of the 7 investigated cases. Altogether, these experiments showed that anti-IgM–mediated protection from ABT-199–induced apoptosis is associated primarily with upregulation of the antiapoptotic protein Mcl-1 and less consistently with downmodulation of the proapoptotic proteins Hrk, Bmf, and BimEL.
Resistance of BCR-stimulated cells to ABT-199 is associated with Mcl-1 upregulation. (A) CLL cells were cultured for 24 hours with or without ABT-199 or ABT-199 + imm-aIgM. ABT-199 (2 nM) was added 3 hours after imm-aIgM. Cellular extracts were analyzed by immunoblotting with the indicated antibodies. Induction of apoptosis was evaluated by PARP cleavage. Actin was used as a loading control. Three representative cases are shown (G329, G333, G372). (B) Correlation between change in Mcl-1 expression and relative protection from ABT-199–induced apoptosis. The latter was defined as the increase in the percentage of viable cells treated with ABT-199 + imm-aIgM relative to ABT-199 alone (%ViableABT-199+imm-aIgM − %ViableABT-199)/%ViableABT-199+imm-aIgM × 100. Change in Mcl-1 expression was defined as fold change in imm-aIgM–stimulated/ABT-199–treated relative to unstimulated/ABT-199–treated CLL B cells. The relationship between Mcl-1 expression and the capacity of imm-aIgM to protect from ABT-199–induced apoptosis was evaluated through Pearson rank correlation. (C) Differences in expression of Bcl-2 family members between unstimulated/ABT-199–treated and imm-aIgM–stimulated/ABT-199–treated cells. Values represent relative protein levels for Mcl-1 (n = 25), Bcl-2 (n = 9), Bcl-xL (n = 9), BimEL (n = 11) and BimL (n = 11), and relative mRNA levels for Bfl-1 (n = 7), Bmf (n = 8), and Hrk (n = 8). Antiapoptotic proteins are shown in the upper panels, and proapoptotic in the lower panels.
Resistance of BCR-stimulated cells to ABT-199 is associated with Mcl-1 upregulation. (A) CLL cells were cultured for 24 hours with or without ABT-199 or ABT-199 + imm-aIgM. ABT-199 (2 nM) was added 3 hours after imm-aIgM. Cellular extracts were analyzed by immunoblotting with the indicated antibodies. Induction of apoptosis was evaluated by PARP cleavage. Actin was used as a loading control. Three representative cases are shown (G329, G333, G372). (B) Correlation between change in Mcl-1 expression and relative protection from ABT-199–induced apoptosis. The latter was defined as the increase in the percentage of viable cells treated with ABT-199 + imm-aIgM relative to ABT-199 alone (%ViableABT-199+imm-aIgM − %ViableABT-199)/%ViableABT-199+imm-aIgM × 100. Change in Mcl-1 expression was defined as fold change in imm-aIgM–stimulated/ABT-199–treated relative to unstimulated/ABT-199–treated CLL B cells. The relationship between Mcl-1 expression and the capacity of imm-aIgM to protect from ABT-199–induced apoptosis was evaluated through Pearson rank correlation. (C) Differences in expression of Bcl-2 family members between unstimulated/ABT-199–treated and imm-aIgM–stimulated/ABT-199–treated cells. Values represent relative protein levels for Mcl-1 (n = 25), Bcl-2 (n = 9), Bcl-xL (n = 9), BimEL (n = 11) and BimL (n = 11), and relative mRNA levels for Bfl-1 (n = 7), Bmf (n = 8), and Hrk (n = 8). Antiapoptotic proteins are shown in the upper panels, and proapoptotic in the lower panels.
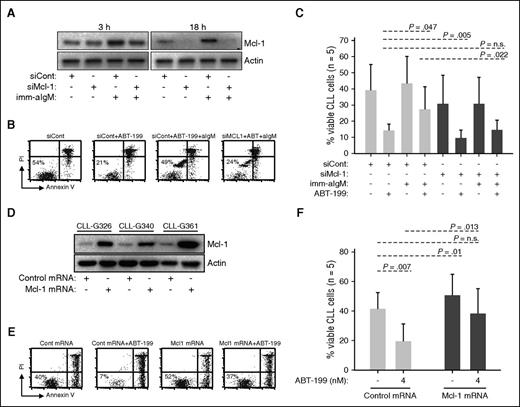
To further explore the role of Mcl-1 in mediating ABT-199 resistance, we performed RNA interference experiments with unstimulated and anti-IgM–stimulated CLL cells. In cells transfected with control siRNA and stimulated with immobilized anti-IgM, an increase in Mcl-1 protein was apparent already following 3 hours of stimulation (Figure 3A). Transfection with Mcl-1 siRNA prevented this increase and, at later time points, reduced Mcl-1 protein expression to undetectable levels. Importantly, Mcl-1 knockdown completely reversed resistance of anti-IgM–stimulated CLL cells to ABT-199, suggesting that induction of this antiapoptotic protein is the primary reason for BCR-mediated protection (Figure 3B-C). Mcl-1 knockdown also sensitized unstimulated CLL cells to ABT-199 (14.2% vs 9.6% for siCont and siMcl-1, respectively), but this effect was less pronounced in comparison with cells stimulated with immobilized anti-IgM (27.4% vs 14.6% for siCont and siMcl-1, respectively).
Mcl-1 mediates ABT-199 resistance. (A-C) CLL cells were transfected with control (siCont) or Mcl-1 (siMcl-1) siRNA, split and cultured with or without imm-aIgM for 3 hours prior to the addition of ABT-199 (2 nM). The levels of Mcl-1 protein were evaluated by immunoblotting of cellular extracts collected 3 and 18 hours after the addition of imm-aIgM. The percentage of viable cells was determined by annexin V/PI staining 20 hours after the addition of imm-aIgM. One representative experiment is shown in panels A and B, respectively. Summary of the data from 5 independent experiments evaluating leukemic cell viability is shown in panel C. Significance of differences in leukemic cell viability was evaluated with the paired Student t test. (D-F) CLL cells were transfected with Mcl-1 or control mRNA. ABT-199 was added after 3 hours, when Mcl-1 protein levels reached maximum, and was removed 3 hours later when Mcl-1 protein levels started to substantially decline (Mcl-1 protein half-life is <1 hour). The ABT-199 concentration in these experiments was increased from 2 nM to 4 nM because of the shorter exposure to the drug. Levels of Mcl-1 protein in CLL cells collected 3 hours postnucleofection are shown in panel D. Analysis of leukemic cell viability in 1 representative sample 20 hours after nucleofection is shown in panel E, whereas summary of data from 5 different experiments evaluating leukemic cell viability is shown in panel F. Significance of differences was evaluated with the paired Student t test.
Mcl-1 mediates ABT-199 resistance. (A-C) CLL cells were transfected with control (siCont) or Mcl-1 (siMcl-1) siRNA, split and cultured with or without imm-aIgM for 3 hours prior to the addition of ABT-199 (2 nM). The levels of Mcl-1 protein were evaluated by immunoblotting of cellular extracts collected 3 and 18 hours after the addition of imm-aIgM. The percentage of viable cells was determined by annexin V/PI staining 20 hours after the addition of imm-aIgM. One representative experiment is shown in panels A and B, respectively. Summary of the data from 5 independent experiments evaluating leukemic cell viability is shown in panel C. Significance of differences in leukemic cell viability was evaluated with the paired Student t test. (D-F) CLL cells were transfected with Mcl-1 or control mRNA. ABT-199 was added after 3 hours, when Mcl-1 protein levels reached maximum, and was removed 3 hours later when Mcl-1 protein levels started to substantially decline (Mcl-1 protein half-life is <1 hour). The ABT-199 concentration in these experiments was increased from 2 nM to 4 nM because of the shorter exposure to the drug. Levels of Mcl-1 protein in CLL cells collected 3 hours postnucleofection are shown in panel D. Analysis of leukemic cell viability in 1 representative sample 20 hours after nucleofection is shown in panel E, whereas summary of data from 5 different experiments evaluating leukemic cell viability is shown in panel F. Significance of differences was evaluated with the paired Student t test.
To provide further evidence that Mcl-1 is directly involved in mediating ABT-199 resistance, we nucleofected primary CLL cells with in vitro–transcribed Mcl-1 mRNA and evaluated leukemic cell viability following a short exposure to the drug. Mcl-1 overexpression resulted in reduced spontaneous CLL cell apoptosis (41.4% vs 50.6% for cells transfected with control and Mcl-1 mRNA, respectively) but had an even greater antiapoptotic effect on cells cultured in the presence of ABT-199 (19.4% vs 38.2% for cells transfected with control and Mcl-1 mRNA, respectively) (Figure 3D-F). Importantly, Mcl-1–transfected cells treated with ABT-199 displayed almost the same viability as untreated cells transfected with control mRNA.
The SYK inhibitor R406 more potently downregulates Mcl-1 than the BTK inhibitor ibrutinib or the PI3Kδ inhibitor idelalisib
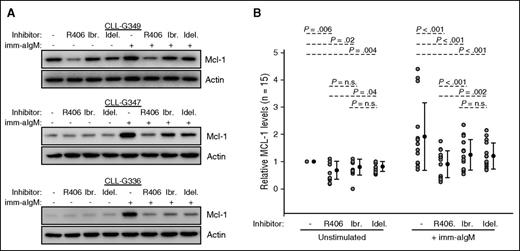
Because the previous experiments showed that BCR-mediated protection from ABT-199 depends on upregulation of Mcl-1, we next compared the capacity of different BCR signaling inhibitors to prevent Mcl-1 induction. We tested the SYK inhibitor R406 (active compound of the oral drug fostamatinib), the BTK inhibitor ibrutinib, and the PI3Kδ inhibitor idelalisib, which are all drugs with proven clinical activity in CLL.25-27 These inhibitors were added to cultured CLL cells 2 hours prior to the addition of immobilized anti-IgM, and the samples were harvested 20 hours later. As shown in Figure 4 and supplemental Figure 3, all 3 inhibitors significantly reduced Mcl-1 expression in anti-IgM–stimulated CLL cells, but with considerable variability between different cases. In 3 of the 12 investigated cases, all 3 inhibitors were equally effective; in 2 cases, idelalisib appeared slightly more effective, whereas in the remaining 7 cases R406 reduced to a considerably greater extent Mcl-1 expression (P < .001 and P = .002 vs ibrutinib and idelalisib, respectively). In addition, R406 reduced to a greater extent Mcl-1 also in unstimulated CLL cells with high basal Mcl-1 levels (sample CLL-G349 in Figure 4 and samples G319, G361, G216, and G372 in supplemental Figure 3). Altogether, these results suggest that R406 more potently downregulates Mcl-1 than ibrutinib or idelalisib.
SYK inhibitor R406 more potently downregulates Mcl-1 than BTK inhibitor ibrutinib or PI3Kδ inhibitor idelalisib. (A) CLL cells were preincubated for 2 hours with 1 μM of the indicated BCR inhibitor and then stimulated with imm-aIgM for additional 20 hours. Cellular extracts were prepared and analyzed by immunoblotting with the indicated antibodies. Three representative experiments of the 15 performed are shown. Additional experiments are shown in supplemental Figure 3. (B) Changes in relative Mcl-1 levels in the 15 investigated samples are plotted on the chart. Analysis of differences between untreated and inhibitor-treated samples was done with the Wilcoxon signed-rank test.
SYK inhibitor R406 more potently downregulates Mcl-1 than BTK inhibitor ibrutinib or PI3Kδ inhibitor idelalisib. (A) CLL cells were preincubated for 2 hours with 1 μM of the indicated BCR inhibitor and then stimulated with imm-aIgM for additional 20 hours. Cellular extracts were prepared and analyzed by immunoblotting with the indicated antibodies. Three representative experiments of the 15 performed are shown. Additional experiments are shown in supplemental Figure 3. (B) Changes in relative Mcl-1 levels in the 15 investigated samples are plotted on the chart. Analysis of differences between untreated and inhibitor-treated samples was done with the Wilcoxon signed-rank test.
SYK inhibitors target Mcl-1 through AKT-dependent and AKT-independent, PKC/GSK-3–dependent mechanisms
To understand the reasons for the different capacity of R406, ibrutinib, and idelalisib to downregulate Mcl-1, we investigated the effects of these inhibitors on downstream signaling molecules regulated by the BCR. We focused particularly on the kinases AKT and GSK-3, because both have been shown to be important regulators of Mcl-1 expression. In particular, AKT has been shown to upregulate Mcl-1 by increasing the translation efficiency of Mcl-1 mRNA, whereas GSK-3 downregulates Mcl-1 by targeting it for proteasomal degradation.22,28-32 As shown in Figure 5A, all 3 BCR signaling inhibitors efficiently blocked anti-IgM–induced activation of AKT, but BCR-induced phosphorylation and inactivation of GSK-3 were blocked to a considerably greater extent by R406 in 9 of the 12 investigated samples (Figure 5A; supplemental Figure 4). A similar effect was observed with GS-9973 (entospletinib), another more selective SYK inhibitor that has also been recently evaluated in clinical trials of CLL (Figure 5B, upper panel).33 This compound displayed the same effects as R406 also in terms of Mcl-1 downregulation (Figure 5B, lower panel). The greater capacity of SYK inhibitors to block GSK-3 phosphorylation was found to be independent of the type of BCR stimulus (soluble or immobilized anti-IgM) and was confirmed by analyzing the expression of β-catenin, another specific GSK-3 substrate (Figures 5C-D; supplemental Figure 4).
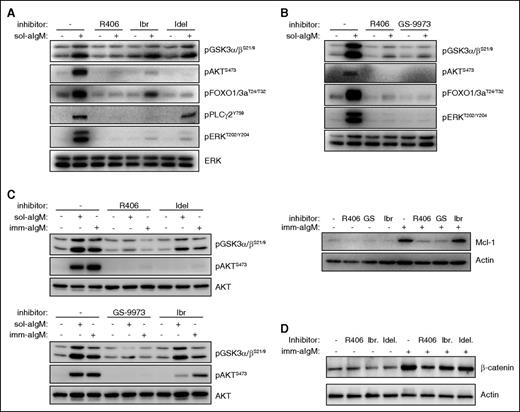
Differential inhibition of downstream BCR signaling pathways by SYK, BTK, and PI3Kδ inhibitors. (A) CLL cells were preincubated for 2 hours with 1 μM of the indicated BCR inhibitor and then stimulated for 10 minutes with 10 μg/mL soluble goat F(ab′)2 anti-human IgM (sol-aIgM). Cellular extracts were prepared and analyzed by immunoblotting with the indicated antibodies. One representative experiment is shown. Ibr, ibrutinib; Idel, idelalisib. (B) CLL cells were preincubated with 1 μM of the indicated inhibitors and stimulated for 10 minutes with sol-aIgM or 24 hours with imm-aIgM. One representative experiment out of 4 is shown. (C) CLL cells were stimulated with sol-aIgM or imm-aIgM in the presence or absence of the indicated inhibitors (1 μM) and then analyzed as in panel A. One representative experiment out of 3 is shown. (D) CLL cells were preincubated with 1 μM of R406, Ibr, or Idel and stimulated for 24 hours with imm-aIgM prior to harvesting and analysis of β-catenin expression.
Differential inhibition of downstream BCR signaling pathways by SYK, BTK, and PI3Kδ inhibitors. (A) CLL cells were preincubated for 2 hours with 1 μM of the indicated BCR inhibitor and then stimulated for 10 minutes with 10 μg/mL soluble goat F(ab′)2 anti-human IgM (sol-aIgM). Cellular extracts were prepared and analyzed by immunoblotting with the indicated antibodies. One representative experiment is shown. Ibr, ibrutinib; Idel, idelalisib. (B) CLL cells were preincubated with 1 μM of the indicated inhibitors and stimulated for 10 minutes with sol-aIgM or 24 hours with imm-aIgM. One representative experiment out of 4 is shown. (C) CLL cells were stimulated with sol-aIgM or imm-aIgM in the presence or absence of the indicated inhibitors (1 μM) and then analyzed as in panel A. One representative experiment out of 3 is shown. (D) CLL cells were preincubated with 1 μM of R406, Ibr, or Idel and stimulated for 24 hours with imm-aIgM prior to harvesting and analysis of β-catenin expression.
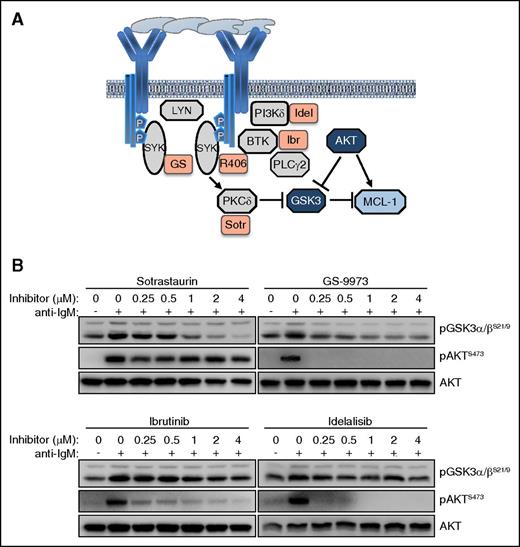
The observation that inhibition of SYK leads to a greater reduction of GSK-3 phosphorylation than inhibition of BTK or PI3Kδ was somewhat unexpected, considering that the investigated GSK-3 inhibitory sites (serine 21 in GSK-3α and serine 9 in GSK-3β) are typically phosphorylated by AKT34 and this kinase was completely inhibited by all 4 investigated drugs. However, previous studies have shown that GSK-3 can also be phosphorylated at these sites by various PKC isoforms,35 and studies in the murine B-cell lymphoma WEHI-231 have shown that anti-IgM–induced phosphorylation of GSK-3 can occur through both AKT-dependent and AKT-independent mechanisms.36 Moreover, previous experiments with human CLL cells have shown that pharmacological inhibition or siRNA-mediated downregulation of SYK induces proteasome-dependent Mcl-1 degradation even in cells that lack any detectable AKT phosphorylation.37 In these cells, SYK inhibition or downregulation was accompanied by reduced PKCδ activity, suggesting that SYK might regulate GSK-3 through direct phosphorylation by PKCδ (Figure 6A). To further explore this possibility, we tested the activity of the pan-PKC inhibitor sotrastaurin in anti-IgM-stimulated CLL cells. Sotrastaurin completely inhibited GSK-3 phosphorylation at concentrations below 1 μM, while having no effect on AKT phosphorylation at even 4 times higher concentrations (Figure 6B). In contrast, ibrutinib and idelalisib completely blocked AKT phosphorylation but had only a modest effect on GSK-3 phosphorylation, whereas GS-9973 was equally effective in inhibiting phosphorylation of both kinases. Together, these results suggest that BCR signals in CLL cells can regulate the activity of GSK-3 through a PKC-dependent AKT-independent pathway, in addition to the canonical AKT-dependent pathway.
The BCR regulates the activity of GSK-3 in CLL cells through an AKT-independent/PKC-dependent pathway. (A) Schematic representation of BCR signaling molecules involved in regulating GSK-3 activity and Mcl-1 expression. (B) Dose titration effects of sotrastaurin (Sotr), GS-9973 (GS), ibrutinib, and idelalisib, on GSK-3 and AKT phosphorylation were evaluated in anti-IgM–stimulated CLL cells. One representative experiment out of 3 performed is shown.
The BCR regulates the activity of GSK-3 in CLL cells through an AKT-independent/PKC-dependent pathway. (A) Schematic representation of BCR signaling molecules involved in regulating GSK-3 activity and Mcl-1 expression. (B) Dose titration effects of sotrastaurin (Sotr), GS-9973 (GS), ibrutinib, and idelalisib, on GSK-3 and AKT phosphorylation were evaluated in anti-IgM–stimulated CLL cells. One representative experiment out of 3 performed is shown.
R406, GS-9973, and idelalisib overcome BCR-mediated ABT-199 resistance more potently than ibrutinib
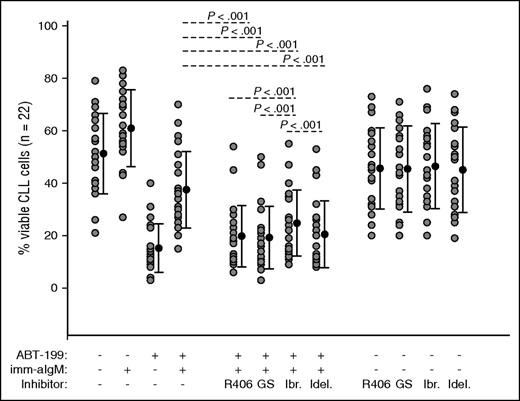
To evaluate the capacity of the different BCR signaling inhibitors to overcome BCR-mediated ABT-199 resistance, we investigated whether R406, GS-9973, ibrutinib, and idelalisib will restore sensitivity of anti-IgM–stimulated CLL cells to ABT-199. CLL cells from 22 patients were pretreated for 2 hours with 1 μM of each inhibitor prior to the addition of immobilized anti-IgM. ABT-199 was added 3 hours later, and cellular viability was analyzed by annexin V/PI staining after overnight culture. As shown in Figure 7, all 4 inhibitors significantly abrogated the protective effect of immobilized anti-IgM. Consistent with the previous data, R406 and GS-9973 restored to a significantly greater extent sensitivity to ABT-199 when compared with ibrutinib. However, neither of the 2 SYK inhibitors was more effective than idelalisib in restoring ABT-199 sensitivity. The absence of a difference between the SYK inhibitors and idelalisib suggests that in some cases Mcl-1–independent mechanisms could be responsible for BCR-mediated ABT-199 resistance.
R406, GS-9973, and idelalisib overcome anti-IgM–mediated ABT-199 resistance more potently than ibrutinib. CLL cells (n = 22) were preincubated for 2 hours with 1 μM of R406, GS-9973, ibrutinib, or idelalisib prior to the addition of imm-aIgM. ABT-199 was added 3 hours later. The percentage of viable cells was determined by annexin V/PI staining and flow cytometry 24 hours after the addition of the SYK inhibitors. Significance of differences between ABT-199/aIgM-cultured cells in the presence or absence of the various inhibitors was evaluated with one-way analysis of variance.
R406, GS-9973, and idelalisib overcome anti-IgM–mediated ABT-199 resistance more potently than ibrutinib. CLL cells (n = 22) were preincubated for 2 hours with 1 μM of R406, GS-9973, ibrutinib, or idelalisib prior to the addition of imm-aIgM. ABT-199 was added 3 hours later. The percentage of viable cells was determined by annexin V/PI staining and flow cytometry 24 hours after the addition of the SYK inhibitors. Significance of differences between ABT-199/aIgM-cultured cells in the presence or absence of the various inhibitors was evaluated with one-way analysis of variance.
Discussion
ABT-199 and its predecessor ABT-263 are extremely cytotoxic for CLL cells in vitro, inducing complete or nearly complete apoptosis at low nanomolar concentrations. However, CLL cells in vivo appear to be substantially more resistant to the cytotoxic effects of these drugs, as evidenced by the relatively low complete response rates observed in recent clinical trials.12,14,38 The greater resistance of CLL cells in vivo is believed to be caused by survival signals that the leukemic cells receive in the lymph node microenvironment, which induce changes in the expression of apoptosis-regulatory proteins not directly targeted by these drugs.39 The existence of such signals is further supported by observations from ongoing clinical trials with ABT-199 in CLL, which demonstrate frequent presence of residual disease in lymph nodes of patients that are otherwise minimal residual disease–negative in the peripheral blood or bone marrow.40,41
A well-characterized survival signal from the lymph node microenvironment that has previously been shown to increase resistance of CLL cells to both ABT-737 and ABT-199 is the CD40 ligand/CD40 interaction, which induces the expression of the antiapoptotic Bcl-2 family proteins Bcl-xL, Bfl-1, and Mcl-1 and downregulates the proapoptotic Bcl-2 family proteins Bim and Noxa.5,7,15,16,42 We now show that BCR engagement represents another important stimulus from the lymph node microenvironment that confers resistance to ABT-199. We also show that this resistance is mediated primarily by induction of Mcl-1, as evidenced by the significant correlation between Mcl-1 protein levels and protection from ABT-199–induced apoptosis, as well as the almost complete lack of protection following Mcl-1 knockdown in primary CLL cells.
Interestingly, although Mcl-1 had previously been shown to confer resistance to ABT-737 and ABT-199 in other hematological malignancies and solid tumors,10,18-20,43,44 its role in mediating resistance in CLL had remained somewhat controversial. For example, in the study of Vogler et al,15 induction of Mcl-1 expression by stimulation with interleukin-4 or interferon-γ was associated with only marginal protection, prompting the authors to conclude that resistance to ABT-737 in CLL is largely Mcl-1 independent. Moreover, in the study of Thijssen et al,16 knockdown of Mcl-1 by RNA interference did not restore susceptibility of CD40-stimulated CLL cells to ABT-199, in contrast to Bcl-xL knockdown. One possible explanation for the discrepancy between these findings and our data is that induction of Mcl-1 by interleukin-4, interferon-γ, or CD40L is relatively modest and below the threshold required for protection. In support of this possibility, we also observed only modest protection in samples with a small increase in Mcl-1 levels following anti-IgM stimulation, suggesting that Mcl-1 can confer ABT-199 resistance in CLL cells only when expressed above a certain threshold. Direct evidence for the latter was provided by overexpressing Mcl-1 in primary CLL cells by mRNA nucleofection, which resulted in significant protection from ABT-199–mediated apoptosis.
Previous studies by us and others had shown that the SYK inhibitors R406 and BAY61-3606 effectively block anti-IgM–mediated induction of Mcl-1 in primary CLL cells.37,45 Because no such data were available for the recently approved BCR inhibitors ibrutinib and idelalisib, we compared the capacity of these agents to target Mcl-1 in anti-IgM–stimulated CLL cells. These experiments demonstrated that ibrutinib and idelalisib can also downmodulate Mcl-1, but less effectively than the SYK inhibitors R406 and GS-9973. Analysis of the effects of these drugs on downstream signaling pathways suggested that the more profound effect of SYK inhibitors on Mcl-1 expression is because of the greater ability of these drugs to block BCR-induced phosphorylation and inactivation of GSK-3, a major negative regulator of Mcl-1. Experiments with the PKC inhibitor sotrastaurin suggested that this difference is because only SYK inhibitors are capable of targeting GSK-3 through both the canonical AKT-dependent and an alternative AKT-independent/PKC-dependent pathway, whereas BTK and PI3Kδ inhibitors target GSK-3 only through the canonical pathway.
It is important to note that the different effects of the BCR signaling inhibitors on Mcl-1 expression and GSK-3 activity were observed at drug concentrations that are clinically achievable and considerably below the maximal plasma concentrations for R406, GS-9973, and idelalisib, suggesting that they are likely to reflect the situation in vivo.25,33,46 The only drug that was used at a higher concentration was ibrutinib, which has a peak plasma concentration that is ∼5 times lower than the one used in our experiments.26 However, the fact that ibrutinib was less effective than R406 or GS-9973 even when used at higher concentrations than those achievable in vivo, suggests even further that SYK inhibitors should be more effective than ibrutinib in downregulating Mcl-1.
Analysis of the capacity of the different BCR signaling inhibitors to overcome anti-IgM–mediated ABT-199 resistance showed significantly greater activity of R406 and GS-9973 in comparison with ibrutinib. However, somewhat unexpectedly, no difference was observed between R406, GS-9973, and idelalisib, despite the generally more potent downregulation of Mcl-1 by the SYK inhibitors. One possible explanation for this discrepancy could be that in some cases GSK-3 and Mcl-1 are regulated only through the canonical AKT-dependent pathway, which could be targeted more effectively by idelalisib than the SYK inhibitors. Consistent with this possibility, we observed that Mcl-1 was more profoundly downregulated by idelalisib than R406 in 2 of the 12 investigated cases (G57 and G329 in supplemental Figure 3). An alternative explanation for the absence of a difference between idelalisib and the SYK inhibitors is that in certain cases Mcl-1–independent mechanisms play a more prominent role in mediating ABT-199 resistance. In support of this possibility, we observed that the levels of BimEL were downmodulated by immobilized anti-IgM in approximately one-third of the investigated cases, consistent with recent findings by others that this protein can be targeted by BCR-derived signals in a subset of CLLs.47 In addition, in more than half of the investigated cases, we observed considerable downmodulation of the proapoptotic proteins Bmf and Hrk. Interestingly, preliminary data in our laboratory suggest that in some cases the changes in the expression of BimEL and Bmf could be more strongly reversed by idelalisib than R406 or GS-9973 (supplemental Figure 5). Although additional experiments will be required to confirm these findings and ascertain the role of these proteins in anti-IgM–mediated ABT-199 resistance, these results indicate that in some patients the greater effects of SYK inhibitors on Mcl-1 expression could be mitigated by a greater capacity of idelalisib to restore expression of BimEL or Bmf.
In summary, the data presented in this study show that BCR signals can protect CLL cells from the BH3 mimetic ABT-199 and that this protection is mediated to a large extent by upregulation of Mcl-1. In addition, they show that SYK inhibitors are more effective than ibrutinib and idelalisib in downmodulating Mcl-1, which translates into a greater capacity of these drugs to overcome ABT-199 resistance, at least with respect to ibrutinib. The lack of a clear advantage with respect to idelalisib suggests that in some cases additional pathways are operating that induce ABT-199 resistance through Mcl-1–independent mechanisms. Further characterization of these pathways is required for the development of tailored combination therapies that will maximally exploit the therapeutic potential of these promising novel agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (project no. AIRC IG_12939) and the Ministero dell'Istruzione, dell'Universita e della Ricerca (ERA-NET TRANSCAN-2 program JTC 2014–project FIRE-CLL). K.B. was supported by fellowships from National Science Center of Poland (PhD fellowship ETIUDA, 2014/12/T/NZ6/00337), Polpharma Scientific Foundation, and Foundation for Polish Science (START scholarship).
Authorship
Contribution: K.B., B.K.S., and D.G.E. designed the study; K.B., B.K.S., S.G., and D.G.E. performed the experiments; I.I., G.P., and L.L. provided patient specimens and clinical data; K.B., B.K.S., and D.G.E. wrote the manuscript; and all authors analyzed data and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitar G. Efremov, International Centre for Genetic Engineering & Biotechnology, Molecular Hematology Unit, Padriciano 99, 34149 Trieste, Italy; e-mail: efremov@icgeb.org.
References
Author notes
K.B. and B.K.S. contributed equally to this study.