In this issue of Blood, Block et al demonstrate that G protein β subunit (Gβ) isoforms are indispensable for chemokine-induced lymphocyte function-associated antigen 1 (LFA-1) integrin activation and neutrophil arrest mediated by Ras-related C3 botulinum toxin substrate 1 (Rac1) and phospholipase C β2/3 (Plcβ2/3).1
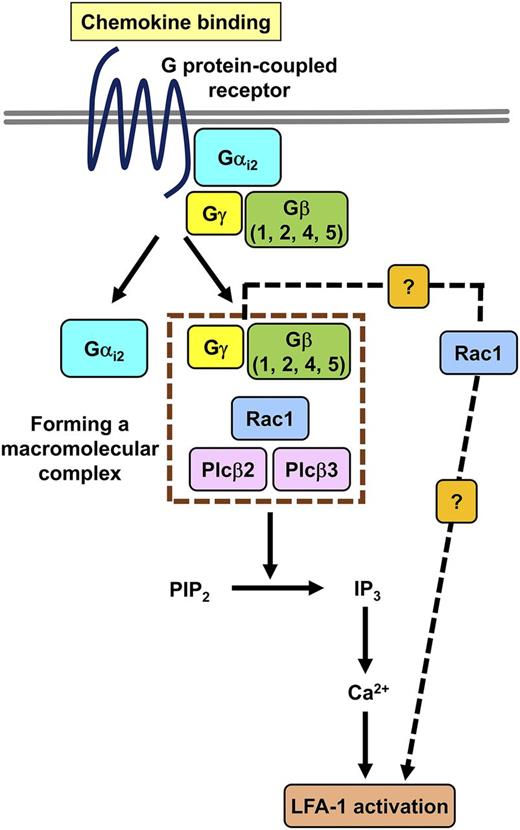
Role of the GPCR Gβ subunit isoforms in chemokine-induced signaling mechanisms leading to LFA-1 activation. Chemokine ligand binding induces the dissociation of the Gα and Gβγ subunits followed by a macromolecular complex formation between the Gβ subunits, Rac1, and Plcβ2/3. The complex initiates the release of IP3 followed by calcium signal, resulting in LFA-1 integrin activation. There might be an alternative signaling pathway (dashed line), in which Rac1 induces LFA-1 activation independently from Plcβ2/3. The figure has been adapted from Figure 6 in the article by Block et al that begins on page 314.
Role of the GPCR Gβ subunit isoforms in chemokine-induced signaling mechanisms leading to LFA-1 activation. Chemokine ligand binding induces the dissociation of the Gα and Gβγ subunits followed by a macromolecular complex formation between the Gβ subunits, Rac1, and Plcβ2/3. The complex initiates the release of IP3 followed by calcium signal, resulting in LFA-1 integrin activation. There might be an alternative signaling pathway (dashed line), in which Rac1 induces LFA-1 activation independently from Plcβ2/3. The figure has been adapted from Figure 6 in the article by Block et al that begins on page 314.
Effective elimination of invading microorganisms requires the recruitment and subsequent activation of leukocytes, in which integrins are central components as major adhesion receptors.2 The activation state of integrins is tightly regulated by intracellular signaling mechanisms termed inside-out signaling, which switches integrins to a high-affinity state. Once integrins are activated, they have the ability to bind integrin ligands and induce integrin outside-in signaling, leading to leukocyte recruitment and activation. The defect of β2 integrin activation results in recurrent bacterial and fungal infections in patients with leukocyte adhesion deficiency type III, indicating the fundamental importance of integrin inside-out signaling in vivo.3,4
The β2 integrin family member LFA-1 is one of the most important leukocyte integrins, and it has 3 possible conformations depending on the activation state. The low-affinity molecule is in a bent, inactive conformation state in resting cells. In the intermediate-conformation state, LFA-1 is extended with a closed headpiece, and in the high-affinity conformation state, the molecule is fully extended with an open headpiece, which allows integrin ligand binding.5,6
It has been well established that G protein-coupled receptor (GPCR)–mediated chemokine signaling effectively induces the high-affinity state of integrins, resulting in integrin activation and ligand binding. Chemokine receptor ligand binding stabilizes the GPCRs in an active conformation leading to the dissociation of G protein Gα and Gβγ subunits and the initiation of further signaling steps.7 Several different Gβγ subunit isoforms are expressed by mammalian cells, but their individual role remains incompletely understood.8 A prior study demonstrated that the Gαi2 subunit plays a predominant role in chemokine-induced neutrophil arrest and recruitment.9 Signaling by the Gβγ subunit isoforms might have also been influenced in those experiments, suggesting the possible role of the Gβγ subunits in the regulation of integrin functions.9
The study by Block et al focused on the role of the Gβ subunit isoforms in chemokine signaling leading to integrin activation and leukocyte recruitment. The authors revealed that all Gβ subunit isoforms expressed by neutrophils (GNB1, GNB2, GNB4, and GNB5) are indispensable for chemokine-induced inositol 1,4,5-trisphosphate (IP3) generation, calcium signal, LFA-1 integrin activation, and neutrophil arrest.1 Downregulation of any single isoform resulted in the absence of integrin activation. Despite the high homology, the Gβ subunit isoforms were not able to compensate for the knockdown of each other, indicating that each isoform plays a nonredundant function in LFA-1 integrin activation.
The study by Block et al indicates that chemokine signaling mediated by Gβγ subunits is at least as important as Gαi2-dependent signaling during inflammation. Furthermore, this is the first study indicating that more Gβ subunits are involved in the same cellular function. This is in contrast to prior studies, in which specialized functions of the individual Gβ subunits were described.10 The previous results indicated that GNB2 was involved in neutrophil chemotaxis, GNB1 knockdown influenced bacterial killing capacity, and the knockdown of both isoforms reduced phagocytosis.10
To characterize the chemokine-induced pathways, Block et al have revealed that GPCR stimulation induced Rac1-dependent activation of both Plcβ2 and Plcβ3. Furthermore, the authors demonstrated that Rac1 activation did not require Plcβ2 or Plcβ3 in the chemokine-induced system but was dependent on Gαi2. The authors also found direct interaction among Rac1, Plcβ2, and Plcβ3 and proposed the possible stimulation-dependent formation of a macromolecular complex with the involvement of Rac1, Plcβ2/3, and Gβ subunits (see figure).
Block et al found that the IP3 levels, calcium signal, neutrophil arrest, and LFA-1 ligand binding and clustering were highly reduced in both Plcβ2- and Plcβ3-deficient neutrophils, whereas Rac1-deficient cells failed to respond. The experiments suggested the additive role of Plcβ2 and Plcβ3 in downstream signaling. According to the proposed model (see figure), the proximal signaling complex initiates further signaling events, leading to IP3 release followed by calcium signal and the subsequent transition of LFA-1 integrin into a high-affinity activated state. It should be noted that Rac1 may also be involved in other signaling pathways, which may induce LFA-1 activation independently from Plcβ2/3. Further studies are needed to characterize the components of these pathways and to define the relative contribution of the possible complementary pathway to LFA-1 activation.
To demonstrate the in vivo relevance of their findings, Block et al used mixed chimeric mice in which Gβ subunit isoform–deficient and control neutrophils can be compared with each other in the same animal. The authors demonstrated that all Gβ subunit isoforms are critical for neutrophil recruitment to the lung in a lipopolysaccharide-induced lung injury model, indicating the importance of the pathway in vivo. Furthermore, the authors demonstrated that Plcβ2, Plcβ3, and Rac1 deficiency reduced the recruitment of neutrophils to the inflamed lung.
The excellent study by Block et al raises several additional questions. Currently it is not clear why the Gβ subunit isoforms show a nonredundant role in chemokine-dependent integrin activation. It is possible that the Gβ subunit isoforms act in the same macromolecular complex. Alternatively, each isoform may be involved in specialized functions, all of which are indispensable for integrin activation. Further investigations are needed to distinguish between these scenarios. The question of whether the activation of other integrins depends on the same signaling machinery should also be addressed. The unexpected role of Rac1 in proximal signaling suggests important questions about the function of the small GTP-binding protein. Revealing novel molecular mechanisms of chemokine-induced inside-out signaling has great importance because modulating integrin activation is a perfect way to regulate leukocyte recruitment and activation at the inflammation site; therefore, components of the integrin inside-out signaling pathways might be important novel therapeutic targets.
In conclusion, Block et al have provided important insights about the role of G protein Gβγ subunits in chemokine-induced signaling through Rac1 and Plcβ2/3 leading to LFA-1 activation in neutrophils.
Conflict-of-interest disclosure: The author declares no competing financial interests.