In this issue of Blood, Milosevic Feenstra et al1 and Cabagnols et al2 report the discovery of heterogeneous novel mutations in MPL and JAK2 genes in 5% to 10% of essential thrombocythemia (ET) and primary myelofibrosis (PMF) patients who lacked what are regarded as classical mutations in these myeloproliferative neoplasms (MPNs) and were thereby considered as having a “triple-negative” (TN) disease. The concept of TN ET and PMF patients was developed after the discovery of calreticulin (CALR) mutations.3,4 The term “triple negativity” was first employed for breast cancer patients who had tumors negative for estrogen or progesterone receptor and HER2 mutations, but it is no longer scientifically correct. TN breast cancers have subsequently been shown to harbor pathogenic mutations in several other genes, including PI3KCA, BRCA1, BRCA2, and PALB2,which are now of increasing importance in clinical management.5 The findings in these 2 manuscripts for TN ET and PMF patients are similarly important and raise several questions for both future research and clinical practice.
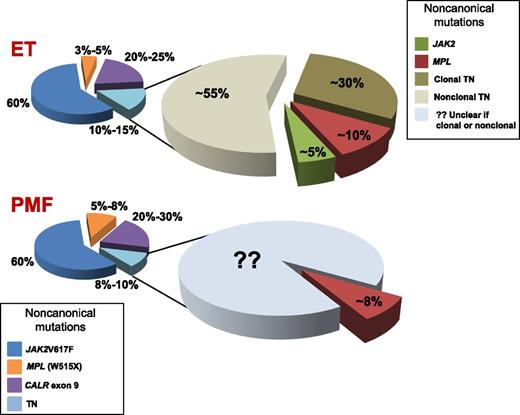
The current genetic landscape concerning phenotypic driver mutations in MPN.
The current genetic landscape concerning phenotypic driver mutations in MPN.
Milosevic Feenstra and colleagues1 began by analyzing tumor cells (granulocytes) and control cells (T lymphocytes) from 8 TN ET and PMF patients subjected to whole-exome sequencing (WES); in 1 patient, a novel somatic mutation at codon 204 of MPL (S204) was discovered. This finding prompted conventional (Sanger) sequencing of the entire coding region of MPL and JAK2 in an additional cohort of 61 TN ET and PMF patients, identifying 5 new MPL mutations, 3 of which were true somatic, 1 was germ line, and 1was not defined because of lack of control tissue, overall accounting for ∼10% of TN patients (see figure). Noncanonical (ie, not V617F) JAK2 mutations were found in 5 of 57 patients; 3 mutations were germ line, and control cells were not available in 2 patients. Due to a negative, or not informative, family history, the patients with germ-line MPL and JAK2 mutations were considered to have sporadic MPN. These newly discovered MPL and JAK2 mutations were functionally validated, even though they appeared to have milder gain-of-function effects on Janus kinase 2/signal transducer and activator of transcription signaling than canonical mutations.
In the second study, Cabagnols et al2 investigated an initial cohort of 17 TN patients with ET by WES targeted on JAK2 and MPL using paired granulocyte and T-cell preparations. Previously described mutations in MPL codons other than codon 515 were found in 3 patients, and 1 patient had a rare mutation at codon 515 (W>R), 1 had a low JAK2V617F allele burden detected, and another had a mutation in a nondriver gene (ie, SH2B3). Four of the remaining 11 patients had evidence of clonal hematopoiesis, based on the detection of heterogeneous, nonrecurrent mutations, whereas in 7 patients, a nonclonal thrombocytosis was diagnosed. Only 1 of 26 additional ET patients analyzed by deep sequencing of MPL and JAK2 was found to have a novel MPLY591 mutation (see figure). Functional analyses qualified MPLS204P and MPLY591N mutations as weak gain-of-function variants.
In his 1951 seminal paper, Dameshek6 wrote, “we find it difficult to draw any clear-cut dividing lines; in fact, so many ‘transition forms’ exist that one may with equal reasonableness call a single condition by at least two different terms.” In 2015, a decade from the original descriptions of JAK2V617F, the MPNs are defined by an increasingly intricate genetic landscape. The articles by Milosevic Feenstra et al1 and Cabagnols et al2 are important because they not only reflect Dameshek’s observations and increase that “intricacy” but also illustrate the limitations of some of the tools that are sometimes taken for granted in both clinical practice and research. For example, the finding of an SRSF2 mutation in addition to MPLS505N in a sample from the study by Cabagnols et al prompted a thorough review of the clinical case that finally led to a reclassification of the diagnosis to myelodysplasia with thrombocytosis (therefore, be certain of the clinical material you are using and be ready to reinterpret initial findings). Additionally, whereas WES and Sanger sequencing initially failed to identify some of the mutations in the cohort of patients, subsequent deep resequencing identified 2 further patients, 1 with a JAK2V617F mutation and another with MPLS204P. These are issues also acknowledged by Milosevic Feenstra and coworkers. Because both studies, based on analyses of T lymphocytes, potentially identified true germ-line mutations, the availability of a different control tissue would be interesting and important.
In addition, there are multiple clinical implications of these findings. The fact that some ET and PMF patients were considered TN solely because the conventional diagnostic tools failed to identify noncanonical mutations in the known phenotypic driver genes sounds reasonable; however, more powerful approaches such as targeted deep sequencing (or even Sanger sequencing) of the entire coding regions of JAK2 and MPL might not be easily transferrable into standard clinical practice. Second, the information that a sizable proportion of TN ET patients do not have a clonal disease might help to explain the overall better prognosis associated with the triple negativity in ET; however, this also raises important issues for the management of such patients for whom cytotoxic treatment may not be appropriate at all. In this regard, although these 2 studies focused mainly on samples from ET patients, with too few PMF patients included to draw any conclusions concerning the implications for TN PMF patients, the prospect of further subdividing this poor-prognosis group would be tantalizing, if it were possible. However, it is not clear how to translate even the data regarding clonality, because none of the methods employed to establish a clonal disease in the 2 articles (ie, WES, comparative genomic hybridization array, or X-linked assays) can reasonably be used in daily practice. Furthermore, these data indirectly suggest that the World Health Organization criteria for the diagnosis of ET, and perhaps PMF, are far from being perfect, because they apparently failed to identify specific patterns helpful to distinguish a true ET from a polyclonal disease. Lastly, if the data presented in these 2 articles are substantiated in further patient cohorts, the proportion of TN ET and PMF patients still considered TN remains high; thus, their underlying pathogenesis remains to be identified.
Conflict-of-interest disclosure: The authors declare no competing financial interests.