Key Points
Gnb isoforms are centrally involved in Rac1-dependent chemokine-induced LFA-1 activation.
Plcβ2 and Plcβ3 function nonredundantly to produce inositol triphosphate with subsequent calcium flux leading to LFA-1 activation.
Abstract
Chemokines are required for leukocyte recruitment and appropriate host defense and act through G protein-coupled receptors (GPCRs), which induce downstream signaling leading to integrin activation. Although the α and β subunits of the GPCRs are the first intracellular molecules that transduce signals after ligand binding and are therefore indispensable for downstream signaling, relatively little is known about their contribution to lymphocyte function-associated antigen 1 (LFA-1) activation and leukocyte recruitment. We used knockout mice and short hairpin RNA to knock down guanine nucleotide binding protein (GNB) isoforms (GNB1, GNB2, GNB4, and GNB5) in HL60 cells and primary murine hematopoietic cells. Neutrophil function was assessed by using intravital microscopy, flow chamber assays, and chemotaxis and biochemistry studies. We unexpectedly discovered that all expressed GNB isoforms are required for LFA-1 activation. Their downregulation led to a significant impairment of LFA-1 activation, which was demonstrated in vitro and in vivo. Furthermore, we showed that GPCR activation leads to Ras-related C3 botulinum toxin substrate 1 (Rac1)-dependent activation of both phospholipase C β2 (Plcβ2) and Plcβ3. They act nonredundantly to produce inositol triphosphate–mediated intracellular Ca2+ flux and LFA-1 activation that support chemokine-induced arrest in vivo. In a complex inflammatory disease model, Plcβ2-, Plcβ3-, or Rac1-deficient mice were protected from lipopolysaccharide-induced lung injury. Taken together, we demonstrated that all Gnb isoforms are required for chemokine-induced downstream signaling, and Rac1, Plcβ2, and Plcβ3 are critically involved in integrin activation and leukocyte arrest.
Introduction
During inflammation, leukocytes get recruited in a coordinated sequence of events. On their way to sites of inflammation, they integrate a variety of inflammatory signals leading to integrin activation.1,2 Neutrophils express two β2 integrins, macrophage-1 antigen (Mac-1) and LFA-1, which support distinct steps during cell arrest and migration on inflamed endothelium. LFA-1 mediates chemokine-induced arrest, whereas Mac-1 is required for postadhesion strengthening and intravascular crawling.3 LFA-1 is expressed at low affinity on unactivated neutrophils and, with activation, a conformational shift in the presentation of its headpiece influences the affinity for ligand binding. Low-affinity integrins persist in a bent conformation, and intermediate affinity is characterized by an extended conformation with a closed headpiece. The high-affinity integrin is characterized by the fully extended conformation of the integrin with an open headpiece.4 Avidity denotes the stability of integrin-mediated cell adhesion and is primarily influenced by membrane rearrangement of integrin receptors from sparse to dense clusters that promote multivalent binding.2,5,6 Chemokine binding to G protein-coupled receptors (GPCRs) rapidly triggers the high-affinity conformation of integrins. Once a sufficient number of integrins adopt a fully activated state, they can bind to their ligands, an important step in leukocyte recruitment.
GPCRs are 7 transmembrane proteins that are identified by the combination of a particular α subunit paired with a βγ complex. Gαi2 was shown to play an important role in neutrophil chemokine-induced arrest,7 whereas the role of the βγ complex in inflammation is only poorly understood. Theoretically, 60 βγ variations can be formed because there are 5 different β and 12 different γ subunits, which are highly conserved among mammalian species.8 Upon stimulation, the α subunit and the βγ complex dissociate and GDP gets released from the α subunit resulting in new capturing of GTP that leads to an active signaling complex.9 Active Gαi subunits can subsequently activate small GTPases. For instance, Gαi-dependent Ras activation subsequently leads to the activation of PI3K by binding to its catalytic subunit.10
The βγ subunit has been shown to interact with a number of molecules, including PI3K isoforms,11 p110β12 , PDZ proteins,13 guanine nucleotide exchange factors such as P-Rex,14 and protein kinase D.15
A prominent downstream effector of GPCR signaling is the family of phospholipase C β (Plcβ). It consists of 4 isoforms, but only Plcβ2 and Plcβ3 are expressed in hematopoietic cells. One study using primary neutrophils demonstrated that the chemokine receptor CXCR2 forms a complex with Plcβ2. Disrupting this complex impairs calcium flux, neutrophil chemotaxis, and migration upon chemokine stimulation.16 However, it is still unknown which molecules are involved in this complex and which molecule directly activates Plcβ isoforms. Biochemical experiments showed that the small GTPase Rac1 is a common binding partner of Plcβ2 and to a lesser extent Plcβ3.17,18 Rac1 is involved in chemoattractant-induced formation and stabilization of the leading edge of migrating cells, such that enhanced activation of Rac1 and Rac2 by ArhGAP15 deficiency can elicit an increase in GPCR-induced migration.19 To date, nothing is known about the role of Rac1 in chemokine-induced integrin activation and neutrophil recruitment in vivo.
In this study, we demonstrate in the human HL60 myeloid cell line as well as in primary murine neutrophils that β1, β2, β4, and β5 subunits are indispensable for GPCR-mediated integrin activation and leukocyte recruitment. We further show that downstream of heterotrimeric G proteins, Rac1 is activated and is required for the activation of Plcβ2 and Plcβ3 leading to the generation of inositol triphosphate (IP3) and subsequent calcium flux. Our findings indicate that chemokine signaling of these Plc isoforms results in additive and nonredundant activation of LFA-1-mediated arrest and leukocyte recruitment in lipopolysaccharide (LPS) -induced lung injury.
Methods
Adhesion flow chamber
Protein G–coated glass capillaries were coated with P-selectin (20 µg/mL), interleukin-8 (IL-8; 50 µg/mL, PeproTech), and immunoglobulin G1 (IgG1; 5 µg/mL) or monoclonal antibody 24 (mAb24; 5 µg/mL). HL60 cells were resuspended in human plasma (5 × 106 cells per milliliter). Flow chambers were perfused with the cell suspension for 2 minutes followed by phosphate-buffered saline for 1 minute. In some experiments, HL60 cells were pre-incubated (30 minutes, room temperature, 1 µM) with transactivator of transcription (TAT) constitutive active Rac1 (TAT-CA Rac1) or TAT wild-type Rac1 (TAT-WT Rac1).20 In representative images, the number of cells per field of view was determined.
Chemotaxis
HL60 cells (4 × 104) or bone marrow–derived neutrophils (1 × 105) were seeded into fibronectin-coated μ-Slides (ibidi) and were incubated for 90 minutes (37°C, 5% CO2). The remaining chambers were filled with medium, and a gradient was applied (IL-8 or CXCL1, 10 µg/mL) according to the manufacturer’s instructions. Chemotactic migration was recorded over a time frame of 4 hours using an Axio Observer (×10 magnification) in an incubation chamber (37°C, 5% CO2), and cell movement was analyzed by using ImageJ software.
ICAM-1 binding assay
Murine bone marrow–derived neutrophils or HL60 cells were isolated and suspended in Hanks balanced salt solution (HBSS; 1 mM CaCl2/MgCl2). Polymorphonuclear neutrophils (PMNs) cells were pre-incubated with anti-CD11b (clone M1/70; 10 μg/mL) to prevent ICAM-1 binding to Mac-1 and were left unstimulated or stimulated with CXCL1 or IL-8 (100 ng/mL, 3 minutes, 37°C; PeproTech), in the presence of ICAM-1/Fc (20 μg/mL; R&D Systems) and allophycocyaninconjugated anti-human IgG1 (Fc-specific; Southern Biotechnology). ICAM-1 binding was measured by using flow cytometry.
Calcium flux
Cells were resuspended (2 × 106/mL) in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) -buffered Tyrode solution,21 incubated with 1 µM Indo-1 (Molecular Probes) for 1 hour at 37°C, washed, and resuspended in HEPES-buffered Tyrode solution (1.25 mM calcium). Measurements were performed by using a FluoroMax-2 spectrofluorometer, visualizing calcium flux after stimulation with 100 ng/mL CXCL1 or IL-8 or 4 µg leukotriene B4 (LTB4) at 37°C.
Murine model of LPS-induced pulmonary inflammation
To induce lung injury and pulmonary inflammation, mice were exposed to nebulized sterile saline as control or to nebulized LPS from Salmonella enteritidis (500 µg/mL; Sigma-Aldrich) for 45 minutes, as previously described.22 Briefly, 24 hours after LPS inhalation, bronchoalveolar lavage (BAL) fluid was collected, leukocyte numbers in the BAL fluid were counted by using Kimura staining, and the percentage of neutrophils was determined by flow cytometry.22
For further information on animals, cell lines and constructs, intravital microscopy, flow cytometry–based assays, LFA-1 clustering, IP3 assay, and biochemical studies, see supplemental Data available on the Blood Web site.
Results
Downregulation of GNB isoforms or GNAI2 strongly affects LFA-1 function
PMNs express four different β subunit GPCR isoforms: GNB1, GNB2, GNB4, and GNB5 (data not shown). We used lentivirally transduced short hairpin RNA (shRNA) against the respective human GNB isoforms to specifically knock down GNB expression in the HL60 promyelocytic cell line. Because the different isoforms display high similarities, we confirmed the specificity of downregulation by using primer-probe combinations for quantitative reverse-transcription polymerase chain reaction specifically for each isoform (Table 1) and investigated whether other isoforms were affected. We have chosen cell lines without any significant compensatory changes in GNB expression for further experiments. We also confirmed the downregulation of the GNB isoforms at the protein level by flow cytometry, which confirmed that this approach did not influence expression of GNAI2 or CXCR2 expression or IL-8 binding to CXCR2 and other surface molecules (Table 2 and supplemental Figure 1A-H).
Relative expression of GNB isoforms in different shRNA-transduced HL60 cell lines
| . | GNB1 (SD) . | GNB2 (SD) . | GNB4 (SD) . | GNB5 (SD) . |
|---|---|---|---|---|
| shGNB1 | 0.37 (± 0.15)* | 1.13 (± 0.19) | 1.07 (± 0.28) | 1.19 (± 0.06) |
| shGNB2 | 1.46 (± 0.29) | 0.05 (± 0.01)* | 0.89 (± 0.13) | 0.93 (± 0.11) |
| shGNB4 | 1.14 (± 0.08) | 1.29 (± 0.09) | 0.12 (± 0.02)* | 1.27 (± 0.21) |
| shGNB5 | 1.15 (± 0.16) | 1.02 (± 0.17) | 0.95 (± 0.11) | 0.10 (± 0.03)* |
| . | GNB1 (SD) . | GNB2 (SD) . | GNB4 (SD) . | GNB5 (SD) . |
|---|---|---|---|---|
| shGNB1 | 0.37 (± 0.15)* | 1.13 (± 0.19) | 1.07 (± 0.28) | 1.19 (± 0.06) |
| shGNB2 | 1.46 (± 0.29) | 0.05 (± 0.01)* | 0.89 (± 0.13) | 0.93 (± 0.11) |
| shGNB4 | 1.14 (± 0.08) | 1.29 (± 0.09) | 0.12 (± 0.02)* | 1.27 (± 0.21) |
| shGNB5 | 1.15 (± 0.16) | 1.02 (± 0.17) | 0.95 (± 0.11) | 0.10 (± 0.03)* |
Values indicate x-fold induction of the expression of the respective molecules in comparison with cells transduced with a nonsilencing scrambled construct. Expression levels were evaluated in a quantitative reverse-transcription polymerase chain reaction using gene-specific primer and probes (n = 4 experiments).
SD, standard deviation.
P < .05 vs gene expression in scrambled cells.
Relative expression of surface molecules in different shRNA-transduced HL60 cell lines
| . | CD11a (SD) . | CD11b (SD) . | CD62L (SD) . | CD162 (SD) . | CXCR2 (SD) . |
|---|---|---|---|---|---|
| shGNB1 | 1.15 (± 0.04) | 1.32 (± 0.23) | 0.90 (± 0.07) | 1.20 (± 0.08) | 1.13 (± 0.39) |
| shGNB2 | 0.77 (± 0.03) | 1.20 (± 0.03) | 0.82 (± 0.11) | 1.37 (± 0.11) | 0.96 (± 0.23) |
| shGNB4 | 0.89 (± 0.07) | 1.02 (± 0.25) | 0.86 (± 0.22) | 0.94 (± 0.32) | 1.06 (± 0.26) |
| shGNB5 | 0.97 (± 0.29) | 0.81 (± 0.06) | 0.98 (± 0.18) | 0.85 (± 0.21) | 0.95 (± 0.28) |
| shGNAI2 | 0.87 (± 0.32) | 1.10 (± 0.12) | 0.92 (± 0.07) | 0.95 (± 0.19) | 1.10 (± 0.14) |
| . | CD11a (SD) . | CD11b (SD) . | CD62L (SD) . | CD162 (SD) . | CXCR2 (SD) . |
|---|---|---|---|---|---|
| shGNB1 | 1.15 (± 0.04) | 1.32 (± 0.23) | 0.90 (± 0.07) | 1.20 (± 0.08) | 1.13 (± 0.39) |
| shGNB2 | 0.77 (± 0.03) | 1.20 (± 0.03) | 0.82 (± 0.11) | 1.37 (± 0.11) | 0.96 (± 0.23) |
| shGNB4 | 0.89 (± 0.07) | 1.02 (± 0.25) | 0.86 (± 0.22) | 0.94 (± 0.32) | 1.06 (± 0.26) |
| shGNB5 | 0.97 (± 0.29) | 0.81 (± 0.06) | 0.98 (± 0.18) | 0.85 (± 0.21) | 0.95 (± 0.28) |
| shGNAI2 | 0.87 (± 0.32) | 1.10 (± 0.12) | 0.92 (± 0.07) | 0.95 (± 0.19) | 1.10 (± 0.14) |
Values indicate x-fold induction of the expression of the different surface molecules in comparison with cells transduced with a nonsilencing scrambled construct. Expression levels were evaluated by flow cytometry. Differences among different cell lines are not statistically significant (n = 4 experiments).
SD, standard deviation.
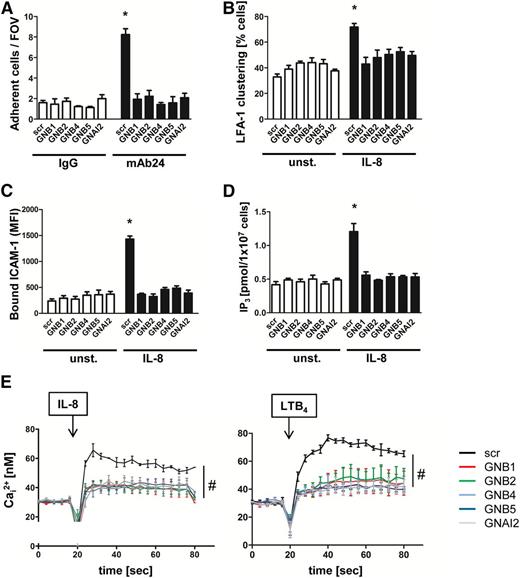
To identify the role of the different β subunits in GPCR-mediated signaling and integrin activation, we examined the various HL60 knockdown cells in adhesion flow chamber experiments.23,24 Flow chambers were co-coated with P-selectin to capture cells, IL-8 to signal activation, and either a control IgG antibody or mAb24, a reporter antibody that recognizes the high affinity conformation of LFA-1. We demonstrated that capture of HL60 knockdown cells on P-selectin, IL-8, and IgG control antibody remained at baseline levels similar to those of control cells (Figure 1A). In contrast, capture was threefold higher in flow chambers coated with P-selectin, IL-8, and mAb24. This increase was significantly decreased to baseline control levels by downregulating each of the β subunits or the Gαi2 subunit (Figure 1A).
Downregulation of GNB isoforms or GNAI2 strongly affects LFA-1 function. HL60 cells transduced with a nonsilencing scrambled (scr) construct or with shRNAs against GNB1, GNB2, GNB4, GNB5, or GNAI2 were used. (A) Number of adherent cells per field of view (FOV) in the adhesion flow chamber. Chambers were coated with P-selectin, IL-8, and either a control IgG antibody or with the reporter mAb24 recognizing high-affinity LFA-1 (n = 4 experiments). *P < .05 vs all other groups. (B) Transduced HL60 cells were left untreated or were stimulated with IL-8, fixed with paraformaldehyde (4%), and stained with anti-CD11a and AF488-labeled secondary antibody to visualize clustered LFA-1 (n = 3 experiments). *P < .05 vs all other groups. (C) Flow cytometry analyzed allophycocyanin (APC)-labeled ICAM-1 binding in unstimulated (unst.) or IL-8 (100 ng/mL) stimulated cells (n = 4 experiments). *P < .05 vs all other groups. (D) Cells were left unstimulated or were stimulated with IL-8 (100 ng/mL, 3 minutes). The reaction was stopped by adding trichloroacetic acid, which was later removed by adding 1,1,2-trichloro-trifluoroethane-trioctylamine. The aqueous IP3-containing supernatant was used for a competitive radioreceptor assay. Bars indicate IP3 concentration in picomoles per 1 × 107 HL60 cells (n = 3 experiments). *P < .05. (E) Concentration of intracellular calcium measured in Indo-1-labeled HL60 cells before and after chemokine stimulation. Arrow indicates IL-8 or LTB4 stimulation (n = 4 experiments). #P < .05 vs all other groups. MFI, mean fluorescent intensity.
Downregulation of GNB isoforms or GNAI2 strongly affects LFA-1 function. HL60 cells transduced with a nonsilencing scrambled (scr) construct or with shRNAs against GNB1, GNB2, GNB4, GNB5, or GNAI2 were used. (A) Number of adherent cells per field of view (FOV) in the adhesion flow chamber. Chambers were coated with P-selectin, IL-8, and either a control IgG antibody or with the reporter mAb24 recognizing high-affinity LFA-1 (n = 4 experiments). *P < .05 vs all other groups. (B) Transduced HL60 cells were left untreated or were stimulated with IL-8, fixed with paraformaldehyde (4%), and stained with anti-CD11a and AF488-labeled secondary antibody to visualize clustered LFA-1 (n = 3 experiments). *P < .05 vs all other groups. (C) Flow cytometry analyzed allophycocyanin (APC)-labeled ICAM-1 binding in unstimulated (unst.) or IL-8 (100 ng/mL) stimulated cells (n = 4 experiments). *P < .05 vs all other groups. (D) Cells were left unstimulated or were stimulated with IL-8 (100 ng/mL, 3 minutes). The reaction was stopped by adding trichloroacetic acid, which was later removed by adding 1,1,2-trichloro-trifluoroethane-trioctylamine. The aqueous IP3-containing supernatant was used for a competitive radioreceptor assay. Bars indicate IP3 concentration in picomoles per 1 × 107 HL60 cells (n = 3 experiments). *P < .05. (E) Concentration of intracellular calcium measured in Indo-1-labeled HL60 cells before and after chemokine stimulation. Arrow indicates IL-8 or LTB4 stimulation (n = 4 experiments). #P < .05 vs all other groups. MFI, mean fluorescent intensity.
Because stable neutrophil arrest mediated by LFA-1 requires not only an upshift in affinity but also in avidity, we investigated LFA-1 clustering after IL-8 stimulation in HL60 cells (Figure 1B). LFA-1 clustering was seen in 70% of IL-8-stimulated cells transduced with the scrambled construct, whereas HL60 cells transduced with shRNAs against the GNB isoforms or GNAI2 showed a significantly reduced level of LFA-1 clustering on activated cells (Figure 1B).
Furthermore, we examined ICAM-1 binding as a readout of LFA-1 affinity and avidity regulation.24 Independent of the transduced construct, unstimulated cells bound equal amounts of ICAM-1 (Figure 1C). After stimulating cells with IL-8, HL60 cells transduced with a scrambled construct bound significantly more ICAM-1 compared with unstimulated cells or HL60 cells in which one β subunit or Gαi2 were downregulated (Figure 1C). To specifically investigate LFA-1 affinity in transduced HL60 cells, we used mAb24 in a flow cytometry–based assay and confirmed that the upregulation of LFA-1 affinity is significantly reduced in HL60 cells in which the GNB isoforms are downregulated (supplemental Figure 1I).
GPCR engagement induces IP3 production, which subsequently leads to increased release of intracellular calcium.25 IP3 and intracellular calcium concentrations in response to IL-8 stimulation were observed to increase in cells with scrambled shRNA (Figure 1D-E). In cell lines transduced with shRNAs against the different GNB isoforms or GNAI2, we did not detect a significant increase of IP3 and intracellular calcium levels after IL-8 stimulation (Figure 1D-E). Likewise, the rapid intracellular calcium flux elicited by LTB4 stimulation was significantly diminished in GNB isoforms or GNAI2 knockdown cells (Figure 1E), expanding the finding to another Gαi-coupled receptor.26
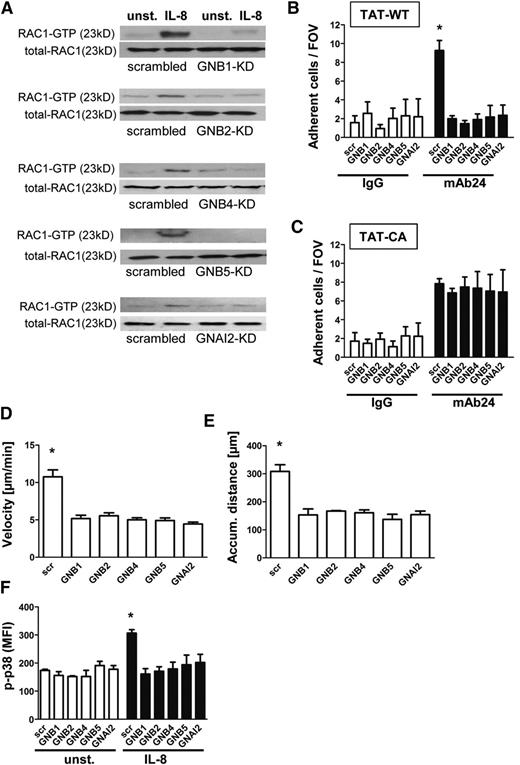
Knockdown of GNB isoforms significantly impairs RAC1-mediated downstream signaling
We next analyzed molecules involved in downstream signaling after GPCR activation and confirmed that RAC1 is activated after IL-8 stimulation in HL60 cells transduced with a nonsilencing construct (Figure 2A). In contrast, cells with reduced expression of GNB1, GNB2, GNB4, GNB5, or GNAI2 failed to activate RAC1 (Figure 2A and supplemental Figure 2A-E). We also demonstrated that RAP1 is not activated in the absence of GNB isoforms (supplemental Figure 2F, representative pull down experiments). To investigate whether this reduced activation of RAC1 is responsible for the observed phenotype, we analyzed HL60 cells pre-incubated with TAT peptides carrying TAT-WT or the RAC1 TAT-CA construct. By using an adhesion flow chamber (Figure 2B), we demonstrated that HL60 cells with downregulated GNB or GNAI2 expression together with a WT-RAC construct failed to adhere to mAb24, indicating that LFA-1 was not upshifted to the high-affinity conformation (Figure 2B). In contrast, pre-incubation with a CA-RAC1 construct resulted in reconstitution of the signaling pathway and consequently LFA-1 activation and cell arrest (Figure 2C). These data indicate a nonredundant function of the different GNB isoforms in GPCR-triggered intracellular signaling because each was necessary for integrin activation and cell adhesion. Analyzing chemotaxis of HL60 along a gradient of IL-8 revealed that each GNB isoform or GNAI2 contributes to the chemotactic migration of cells. Cells with downregulated expression of these molecules migrated at lower velocity (Figure 2D) and for shorter distances (Figure 2E) because of a reduced persistence during chemotaxis (data not shown) in comparison with HL60 transduced with a nonsilencing construct.
Knockdown of GNB isoforms significantly impairs RAC1-mediated downstream signaling. (A) Representative western blots of scrambled or shRNA-transduced HL60 cells, which were left untreated or stimulated with IL-8 (100 ng/mL), lysed, and used to pull down GTP-bound active RAC1. Total RAC1 served as loading control (n = 3 experiments). (B) Number of adherent cells per field of view in the adhesion flow chamber after pre-incubation with TAT-WT RAC or (C) TAT-CA RAC construct (n = 4 experiments). *P < .05 vs all other groups. (D-E) Chemotactic migration of HL60 cells transduced with a scrambled construct or different shRNAs (n = 3 experiments; >60 cells). *P < .05 vs all groups. (D) Migration velocity and (E) accumulated (accum.) distance toward the applied IL-8 gradient in the ibidi μ-Slide. (F) Transduced HL60 cells were left unstimulated or incubated with IL-8 (100 ng/mL, 3 minutes, 37°C), fixed, and permeabilized to stain intracellular p-p38 MAPK. Staining was analyzed by using flow cytometry (n = 3 experiments). *P < .05 vs all other groups.
Knockdown of GNB isoforms significantly impairs RAC1-mediated downstream signaling. (A) Representative western blots of scrambled or shRNA-transduced HL60 cells, which were left untreated or stimulated with IL-8 (100 ng/mL), lysed, and used to pull down GTP-bound active RAC1. Total RAC1 served as loading control (n = 3 experiments). (B) Number of adherent cells per field of view in the adhesion flow chamber after pre-incubation with TAT-WT RAC or (C) TAT-CA RAC construct (n = 4 experiments). *P < .05 vs all other groups. (D-E) Chemotactic migration of HL60 cells transduced with a scrambled construct or different shRNAs (n = 3 experiments; >60 cells). *P < .05 vs all groups. (D) Migration velocity and (E) accumulated (accum.) distance toward the applied IL-8 gradient in the ibidi μ-Slide. (F) Transduced HL60 cells were left unstimulated or incubated with IL-8 (100 ng/mL, 3 minutes, 37°C), fixed, and permeabilized to stain intracellular p-p38 MAPK. Staining was analyzed by using flow cytometry (n = 3 experiments). *P < .05 vs all other groups.
To confirm whether the intracellular signaling cascade after IL-8 stimulation is also disturbed, we conducted intracellular phosphorylated p38 (p-p38) MAPK staining and analyzed the staining with flow cytometry (Figure 2F). p38 MAPK was activated after IL-8 stimulation in cells transduced with the scrambled construct, whereas cells transduced with shRNAs against the different GNB isoforms or GNAI2 displayed no significant increase of p38 MAPK phosphorylation (Figure 2F).
Knockdown of Gnb isoforms in primary neutrophils significantly abolishes chemokine-induced arrest in vivo and neutrophil migration in vitro
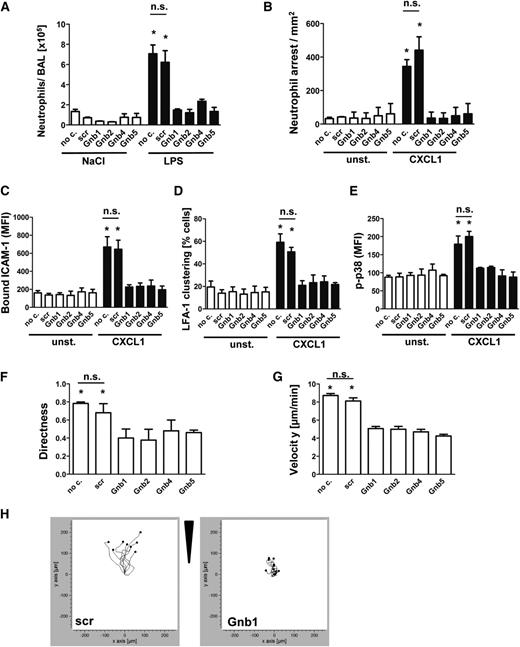
To confirm the physiological relevance of our results obtained with the human HL60 cell line, we transduced murine hematopoietic stem cells with shRNAs against the various murine Gnb isoforms and transplanted these cells into lethally irradiated WT mice. Subsequently, these mixed chimeric mice were exposed to LPS-induced lung injury to investigate neutrophil recruitment into the lung (Figure 3A). Transduced neutrophils were observed to express levels of maturation markers and adhesion molecules equivalent to those of the scrambled control construct (supplemental Figure 3A-E).
Knockdown of Gnb isoforms in primary neutrophils significantly abolishes chemokine-induced arrest in vivo and neutrophil migration in vitro. Retrovirally transduced hematopoietic stem cells with shRNAs against Gnb1, Gnb2, Gnb4, and Gnb5 or with a scrambled construct were transplanted into lethally irradiated WT recipients. (A) Mixed chimeric mice were exposed to nebulized saline or LPS to induce lung injury. Data indicate number of neutrophils in the BAL 24 hours after inhalation of NaCl (left panel) or LPS (right panel) (n = 3). *P < .05 vs all other groups. (B) Mixed chimeric mice were used to investigate CXCL1-induced arrest in the cremaster muscle (n = 3). *P < .05 vs all other groups. (C) Purified bone marrow–derived neutrophils were used for an ICAM-1-binding assay. During flow cytometry acquisition, cells were gated into GFP-positive (shRNA-containing) and GFP-negative (WT) cell populations and analyzed for CXCL1-induced increase of bound APC-labeled ICAM-1. Data represent MFI of APC in nontransduced and transduced cells, both unstimulated and stimulated (n = 4 experiments). *P < .05 vs all other groups. (D) Isolated PMNs from mixed chimeric mice were left untreated or were stimulated with CXCL1, fixed with paraformaldehyde (4%), and stained with anti-CD11a and AF488-labeled secondary antibody to visualize clustered LFA-1 (n = 3 experiments). *P < .05 vs all other groups. (E) Purified bone marrow–derived neutrophils were left unstimulated or incubated with CXCL1 (100 ng/mL, 3 minutes, 37°C), fixed, and permeabilized to stain intracellular p-p38 MAPK. MFI of bound AF647-labeled p38 MAPK antibody was analyzed by using flow cytometry gating into GFP-positive and GFP-negative populations (n = 3 experiments). *P < .05 vs all other groups. (F-H) Chemotactic migration of purified bone marrow–derived neutrophils of mixed chimeric mice (n = 3 experiments; >40 cells). *P < .05 vs all groups. (F) Migration directness and (G) migration velocity toward the applied CXCL1 gradient in the ibidi μ-Slide. (H) Representative migration plots of cells transduced with a scrambled construct or with an shRNA against 1 Gnb isoform. Arrow indicates applied CXCL1 gradient. no c., no construct; n.s., not significant.
Knockdown of Gnb isoforms in primary neutrophils significantly abolishes chemokine-induced arrest in vivo and neutrophil migration in vitro. Retrovirally transduced hematopoietic stem cells with shRNAs against Gnb1, Gnb2, Gnb4, and Gnb5 or with a scrambled construct were transplanted into lethally irradiated WT recipients. (A) Mixed chimeric mice were exposed to nebulized saline or LPS to induce lung injury. Data indicate number of neutrophils in the BAL 24 hours after inhalation of NaCl (left panel) or LPS (right panel) (n = 3). *P < .05 vs all other groups. (B) Mixed chimeric mice were used to investigate CXCL1-induced arrest in the cremaster muscle (n = 3). *P < .05 vs all other groups. (C) Purified bone marrow–derived neutrophils were used for an ICAM-1-binding assay. During flow cytometry acquisition, cells were gated into GFP-positive (shRNA-containing) and GFP-negative (WT) cell populations and analyzed for CXCL1-induced increase of bound APC-labeled ICAM-1. Data represent MFI of APC in nontransduced and transduced cells, both unstimulated and stimulated (n = 4 experiments). *P < .05 vs all other groups. (D) Isolated PMNs from mixed chimeric mice were left untreated or were stimulated with CXCL1, fixed with paraformaldehyde (4%), and stained with anti-CD11a and AF488-labeled secondary antibody to visualize clustered LFA-1 (n = 3 experiments). *P < .05 vs all other groups. (E) Purified bone marrow–derived neutrophils were left unstimulated or incubated with CXCL1 (100 ng/mL, 3 minutes, 37°C), fixed, and permeabilized to stain intracellular p-p38 MAPK. MFI of bound AF647-labeled p38 MAPK antibody was analyzed by using flow cytometry gating into GFP-positive and GFP-negative populations (n = 3 experiments). *P < .05 vs all other groups. (F-H) Chemotactic migration of purified bone marrow–derived neutrophils of mixed chimeric mice (n = 3 experiments; >40 cells). *P < .05 vs all groups. (F) Migration directness and (G) migration velocity toward the applied CXCL1 gradient in the ibidi μ-Slide. (H) Representative migration plots of cells transduced with a scrambled construct or with an shRNA against 1 Gnb isoform. Arrow indicates applied CXCL1 gradient. no c., no construct; n.s., not significant.
This method allowed us to compare neutrophil recruitment of WT cells and shRNA-transduced cells under identical conditions in vivo. After mice were exposed to nebulized saline, the number of neutrophils in the bronchoalveolar space in all mice was low. In response to inhalation of LPS, we observed that scrambled construct neutrophils were recruited nearly as effectively as untransduced neutrophils (no construct), whereas neutrophils transduced with Gnb shRNAs were significantly less recruited (Figure 3A).
In addition, we performed CXCL1-induced arrest in the cremaster muscle in mixed chimeric mice to assess microvascular recruitment efficiency (Figure 3B). Untransduced WT leukocytes and leukocytes that were transduced with a scrambled construct adhered immediately after CXCL1 injection. In contrast, leukocytes transduced with shRNAs against the various Gnb isoforms failed to adhere to the vessel wall (Figure 3B).
To investigate LFA-1 function, we analyzed ex vivo ICAM-1 binding capacity. Mice were euthanized, and neutrophils were isolated and assayed for ICAM-1 binding.24 The advantage of this procedure is that nontransduced cells served as a positive control within the same sample receiving the same stimuli and exactly the same treatment. Cells transduced with nonsilencing scrambled control shRNA showed an increase of ICAM-1 binding after CXCL1 stimulation similar to that of the nontransduced cells within the same sample (Figure 3C). In contrast, cells transduced with shRNAs against Gnb1, Gnb2, Gnb4, or Gnb5 (Figure 3C) registered significantly less ICAM-1 binding upon stimulation compared with their nontransduced equivalents within the same sample. To investigate LFA-1 avidity, we analyzed LFA-1 clustering after CXCL1 stimulation (Figure 3D). We observed an increase only in the fraction of cells with clustered LFA-1 in nontransduced cells or those transduced with a scrambled construct. Cells transduced with Gnb shRNAs did not significantly increase LFA-1 clusters compared with unstimulated cells (Figure 3D).
To confirm that GPCR-induced signaling is impaired in these mice, we analyzed phosphorylation of p38 MAPK (Figure 3E). In control cells (no construct/scrambled), phosphorylation of p38 MAPK was significantly elevated after CXCL1 stimulation, whereas no significant increase in the phosphorylation of p38 MAPK was detected in cells transduced with the various Gnb shRNAs after stimulation (Figure 3E). We also investigated whether deficiency of Gnb isoforms impairs chemotactic migration toward a CXCL1 gradient. Again, we observed that all isoforms are involved in this process. Cells transduced with the various shRNAs migrated with impaired persistence (Figure 3F) and at lower velocities (Figure 3G) compared with untransduced cells or those transfected with a nonsilencing construct. Figure 3H shows representative migration plots of cell movement of control cells and shRNA-transduced cells.
GPCR signaling activates Plcβ2 and Plcβ3 in an Rac1-dependent manner
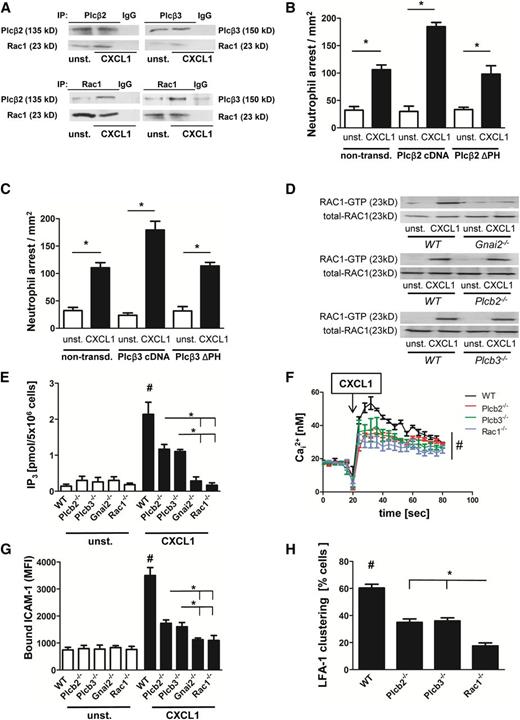
Phospholipase C (PLC) is involved in the increase of intracellular calcium flux after GPCR engagement.25 However, it is still unknown how PLC is activated and which PLC isoform is responsible for the increase of the intracellular calcium concentration after GPCR engagement in neutrophils. Because Rac1 is known to interact with Plcβ2 and Plcβ3, we investigated the role of these molecules in GPCR-mediated activation of LFA-1. By using co-immunoprecipitation, we demonstrated in primary mouse neutrophils that Rac1 complexes with Plcβ2 and Plcβ3 in unactivated cells, and its association increases upon CXCL1 stimulation (Figure 4A). To confirm the specificity of this association, we performed reciprocal immunoprecipitation of Rac1 and densitometric analysis (Figure 4A and supplemental Figure 4A-B). Data indicate that Rac1, Plcβ2, Plcβ3, and Gnb isoforms interact with each other to form a complex that is increased with CXCL1 signaling in primary neutrophils (supplemental Figure 4C). Published studies using in vitro systems have revealed that Rac1 can bind Plcβ isoforms via their pleckstrin homology (PH) domain.17 By using a pulldown assay, we also demonstrated that the PH domain of Plcβ isoforms is able to bind to Rac1 (supplemental Figure 4D). To investigate whether the PH domains of Plcβ2 and Plcβ3 are necessary for downstream signaling, integrin activation, and leukocyte recruitment, we performed in vivo reconstitution experiments with mutant constructs of Plcβ2 and Plcβ3 lacking their PH domains. These constructs were expressed in primary knockout cells, and mixed chimeric mice were generated. Investigating chemokine-induced arrest in these mice revealed that full-length complementary DNA constructs of both Plcβ2 (Figure 4B) and Plcβ3 (Figure 4C) restored Plcβ function. In contrast, ΔPH constructs of Plcβ2 (Figure 4B) or Plcβ3 (Figure 4C) failed to compensate Plcβ deficiency.
GPCR signaling activates Plcβ2 and Plcβ3 in a Rac1-dependent manner. (A) Co-immunoprecipitation (co-IP) of Plcβ2 or Plcβ3 with Rac1. Bone marrow–derived WT neutrophils were left untreated or were stimulated with CXCL1 (100 ng/mL, 3 minutes, 37°C), lysed, and used for precipitation with Plcβ2 or Plcβ3 antibody and were blotted and incubated with Rac1 antibody (upper panel) or (lower panel) used for precipitation with Rac1 and then blotted and incubated with Plcβ2 or Plcβ3 antibody (n = 3 experiments for both). For densitometric analysis, see supplemental Figure 4A-B. (B-C) Number of arrested cells per square millimeter after injection of 500 ng CXCL1 in cremaster muscles of mice transplanted with retrovirally transduced Plcβ-knockout (KO) bone marrow. Chemokine-induced arrest of (B) Plcβ2-KO bone marrow nontransduced (non-transd.) or transduced with Plcβ2-complementary DNA (cDNA) or Plcβ2-ΔPH and (C) Plcβ3-KO bone marrow nontransduced or transduced with Plcβ3-cDNA or Plcβ3-ΔPH. (D) Representative western blots of WT, Gnai2−/−, Plcβ2−/−, or Plcβ3−/− neutrophils, which were left untreated or stimulated with CXCL1 (100 ng/mL), lysed, and used to pull down GTP-bound active RAC1. Total RAC1 serves as loading control (n = 3 experiments). (E) Intracellular concentrations of IP3 before and after stimulating cells with CXCL1 (100 ng/mL, 3 minutes; n = 3 experiments). #P < .05 WT vs all other groups; *P < .05, Plcβ2−/− vs Gnai2−/− and Rac1−/−, and Plcβ3−/− vs Gnai2−/− and Rac1−/−. (F) Bone marrow–derived Indo-1-labeled neutrophils from WT, Plcb2−/−, Plcb3−/−, and Rac1−/− mice were investigated for intracellular calcium levels before and after stimulation with CXCL1. Arrow indicates CXCL1 treatment (100 ng/mL; n = 4 experiments). #P = .05. (G) Flow cytometry was used to analyze APC-labeled ICAM-1 binding in unstimulated or CXCL1 (100 ng/mL, 3 minutes) stimulated bone marrow–derived neutrophils (n = 3 experiments). #P < .05 WT vs all other groups; *P < .05 Plcβ2−/− vs Gnai2−/− and Rac1−/−, and Plcβ3−/− vs Gnai2−/− and Rac1−/−. (H) Percentage of clustered cells in the inflamed cremaster muscle (tumor necrosis factor-α, 2 hours; n = 4). #P < .05 WT vs all other groups. *P < .05 Plcβ2−/− vs Rac1−/−, and Plcβ3−/− vs Rac1−/−.
GPCR signaling activates Plcβ2 and Plcβ3 in a Rac1-dependent manner. (A) Co-immunoprecipitation (co-IP) of Plcβ2 or Plcβ3 with Rac1. Bone marrow–derived WT neutrophils were left untreated or were stimulated with CXCL1 (100 ng/mL, 3 minutes, 37°C), lysed, and used for precipitation with Plcβ2 or Plcβ3 antibody and were blotted and incubated with Rac1 antibody (upper panel) or (lower panel) used for precipitation with Rac1 and then blotted and incubated with Plcβ2 or Plcβ3 antibody (n = 3 experiments for both). For densitometric analysis, see supplemental Figure 4A-B. (B-C) Number of arrested cells per square millimeter after injection of 500 ng CXCL1 in cremaster muscles of mice transplanted with retrovirally transduced Plcβ-knockout (KO) bone marrow. Chemokine-induced arrest of (B) Plcβ2-KO bone marrow nontransduced (non-transd.) or transduced with Plcβ2-complementary DNA (cDNA) or Plcβ2-ΔPH and (C) Plcβ3-KO bone marrow nontransduced or transduced with Plcβ3-cDNA or Plcβ3-ΔPH. (D) Representative western blots of WT, Gnai2−/−, Plcβ2−/−, or Plcβ3−/− neutrophils, which were left untreated or stimulated with CXCL1 (100 ng/mL), lysed, and used to pull down GTP-bound active RAC1. Total RAC1 serves as loading control (n = 3 experiments). (E) Intracellular concentrations of IP3 before and after stimulating cells with CXCL1 (100 ng/mL, 3 minutes; n = 3 experiments). #P < .05 WT vs all other groups; *P < .05, Plcβ2−/− vs Gnai2−/− and Rac1−/−, and Plcβ3−/− vs Gnai2−/− and Rac1−/−. (F) Bone marrow–derived Indo-1-labeled neutrophils from WT, Plcb2−/−, Plcb3−/−, and Rac1−/− mice were investigated for intracellular calcium levels before and after stimulation with CXCL1. Arrow indicates CXCL1 treatment (100 ng/mL; n = 4 experiments). #P = .05. (G) Flow cytometry was used to analyze APC-labeled ICAM-1 binding in unstimulated or CXCL1 (100 ng/mL, 3 minutes) stimulated bone marrow–derived neutrophils (n = 3 experiments). #P < .05 WT vs all other groups; *P < .05 Plcβ2−/− vs Gnai2−/− and Rac1−/−, and Plcβ3−/− vs Gnai2−/− and Rac1−/−. (H) Percentage of clustered cells in the inflamed cremaster muscle (tumor necrosis factor-α, 2 hours; n = 4). #P < .05 WT vs all other groups. *P < .05 Plcβ2−/− vs Rac1−/−, and Plcβ3−/− vs Rac1−/−.
To investigate whether Rac1 is upstream or downstream of the Plcβ isoforms, we performed Rac1 pulldown assays. We found that Rac1 was activated after CXCL1 stimulation in WT PMNs, but not in Gnai2−/− PMNs (Figure 4D). In contrast, Rac1 was activated in PMNs deficient for Plcβ2 and Plcβ3 (Figure 4D), indicating that neither Plcβ2 nor Plcβ3 are involved in activating Rac1, whereas upstream signaling of Gαi2 is associated with its activation.
Next, we analyzed the role of Plcb2−/−, Plcb3−/−, Rac1−/−, and Gnai2−/− mice in modulating IP3 and intracellular calcium levels downstream of chemokine stimulation. By using a radioactive binding assay in which radiolabeled tritiated [3H]IP3 competes with unlabeled intracellular IP3, we investigated the increase of intracellular IP3 in the various knockdown cell lines after IL-8 stimulation. We observed that Plcβ2- and Plcβ3-deficient cells displayed a reduced increase of IP3 generation in comparison with WT PMNs, whereas Rac1- and Gnai2-deficient PMNs failed to generate an increase in IP3 after CXCL1 stimulation (Figure 4E). Accordingly, WT PMNs showed the highest calcium flux after CXCL1 stimulation (Figure 4F), whereas Plcb2−/−, Plcb3−/−, and Rac1-deficient PMNs had a significantly reduced calcium flux (Figure 4F).
To investigate LFA-1 activation in the different knockout mouse lines, we performed an ICAM-1 binding assay. Upon stimulation, WT PMNs showed a significant increase in ICAM-1 binding (Figure 4G). Plcβ2- and Plcβ3-deficient cells showed reduced ICAM-1 binding compared with WT-PMNs, whereas cells deficient for Gnai2 or Rac1 displayed no increase in ICAM-1 binding after CXCL1 stimulation (Figure 4G). To demonstrate that LFA-1 clustering as a measure of avidity is also affected, we analyzed LFA-1 clustering in vivo in the inflamed cremaster muscle. Two hours after tumor necrosis factor-α application, we injected a fluorescence-labeled anti-LFA-1 antibody and observed that LFA-1 clustering was significantly reduced in Plcβ2- and Plcβ3-deficient mice compared with WT mice (Figure 4H). Again, Rac1-deficient mice displayed even less LFA-1 clustering (Figure 4H). These data demonstrate that Rac-1 is required for the activation of Plcβ isoforms and the subsequent IP3 generation and calcium flux after GPCR engagement.
To test whether Rac2 is involved in the regulation of intracellular calcium levels and integrin activation after chemokine stimulation, we used the Rac inhibitor NSC 23766, which blocks Rac1 and Rac2 in Rac1−/− and WT neutrophils. Blocking Rac reduced ICAM-1 binding and increase in intracellular calcium levels after chemokine stimulation (supplemental Figure 4E-F). However, using the Rac inhibitor in Rac1-deficient neutrophils did not further diminish ICAM-1 binding or calcium flux significantly, suggesting that Rac2 is not involved in the regulation of intracellular calcium levels and integrin activation after chemokine stimulation (supplemental Figure 4E-F).
Plcβ and Rac1 deficiency leads to reduced chemokine-induced arrest and protects from LPS-induced lung injury
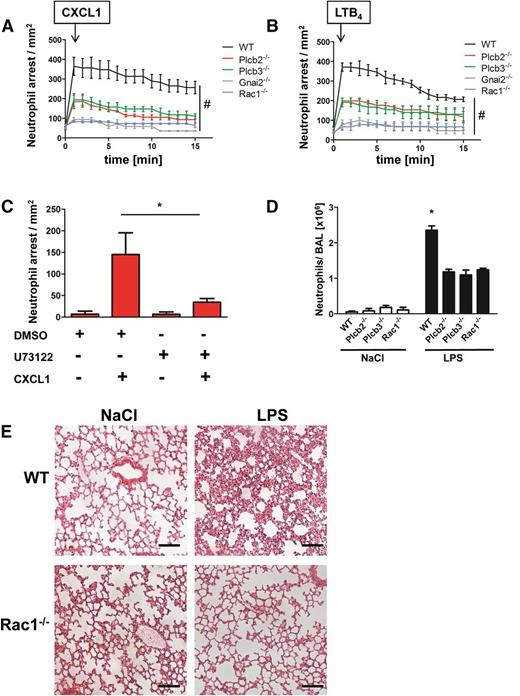
To investigate the role of the two Plcβ isoforms and Rac1 in leukocyte adhesion in vivo, we performed intravital microscopy of the cremaster muscle using WT and different chimeric gene–deficient knockout mouse lines. We first confirmed that the various mouse lines had a normal number of leukocytes and neutrophils in the blood (supplemental Figure 5A-B). We demonstrated that both Plcβ2- and Plcβ3-deficient mice showed a reduced number of stably arrested cells after CXCL1 injection compared with WT mice (Figure 5A). We also observed an even stronger deficiency in chemokine-induced arrest in mice deficient for Rac1 or Gnai2 (Figure 5A). Similar results were obtained by using LTB4 as the stimulating reagent (Figure 5B). To confirm that the capacity of leukocytes to stably adhere is the result of the remaining Plcβ isoform, we performed chemokine-induced arrest in Plcβ2-deficient mice after pretreatment with the PLC inhibitor U73122 or with vehicle control dimethylsulfoxide (Figure 5C). After treatment with the inhibitor, the immediate chemokine-induced arrest was abolished in these mice, confirming that residual PLC activity is responsible for the intermediate phenotype observed in Figure 5A. These experiments revealed that chemokine-induced arrest is strongly affected in these mouse lines, suggesting that Gαi2, Rac1, Plcβ2, and Plcβ3 are involved in LFA-1 activation in vivo.
Plcβ and Rac1 deficiency leads to reduced chemokine arrest and protects from LPS-induced lung injury. (A-B) WT mice transplanted with WT, Plcb2−/−, Plcb3−/−, Gnai2−/−, and Rac1−/− bone marrow cells were used to determine chemokine-induced arrest in the cremaster muscle. Arrested cells were counted after injection of 500 ng (A) CXCL1 or (B) LTB4 over 15 minutes. (C) Chemokine-induced arrest of Plcb2−/− mice pretreated with dimethylsulfoxide (DMSO) or with PLC inhibitor U73122. Arrested cells were determined before and after injection of 500 ng CXCL1 (n = 3). *P < .05. (D) WT, Plcb2−/−, Plcb3−/−, or Rac1−/− mice were exposed to nebulized NaCl or LPS; 24 hours later, mice were euthanized and the bronchoalveolar fluid was collected and analyzed for neutrophil recruitment (n = 4). *P < .05 vs all other groups. (E) Representative images of hematoxylin and eosin (H&E)–stained formalin-fixed paraffin-embedded lung sections (slices 5 µm thick) from WT and Rac1−/− mice, which were exposed to nebulized saline or LPS. Images were acquired by using a Zeiss LSM510 (×20 magnification).
Plcβ and Rac1 deficiency leads to reduced chemokine arrest and protects from LPS-induced lung injury. (A-B) WT mice transplanted with WT, Plcb2−/−, Plcb3−/−, Gnai2−/−, and Rac1−/− bone marrow cells were used to determine chemokine-induced arrest in the cremaster muscle. Arrested cells were counted after injection of 500 ng (A) CXCL1 or (B) LTB4 over 15 minutes. (C) Chemokine-induced arrest of Plcb2−/− mice pretreated with dimethylsulfoxide (DMSO) or with PLC inhibitor U73122. Arrested cells were determined before and after injection of 500 ng CXCL1 (n = 3). *P < .05. (D) WT, Plcb2−/−, Plcb3−/−, or Rac1−/− mice were exposed to nebulized NaCl or LPS; 24 hours later, mice were euthanized and the bronchoalveolar fluid was collected and analyzed for neutrophil recruitment (n = 4). *P < .05 vs all other groups. (E) Representative images of hematoxylin and eosin (H&E)–stained formalin-fixed paraffin-embedded lung sections (slices 5 µm thick) from WT and Rac1−/− mice, which were exposed to nebulized saline or LPS. Images were acquired by using a Zeiss LSM510 (×20 magnification).
To investigate the role of Plcβ2, Plcβ3, and Rac1 in a complex disease model, we analyzed the recruitment of neutrophils into the bronchoalveolar space in WT, Plcβ2-, Plcβ3-, and Rac1-deficient chimeric mice (Figure 5D). Mice were subjected to saline or LPS inhalation, and the number of neutrophils in the BAL was determined 24 hours later. Saline inhalation led to only small numbers of recruited cells in WT and knockout mice. In contrast, after LPS inhalation, chimeric knockout mice displayed significantly fewer recruited neutrophils in comparison with WT mice (Figure 5D). A representative example of leukocyte recruitment into the lung and the concurrent damage of the lung architecture of WT mice in comparison with Rac1-deficient mice is shown in Figure 5E.
Discussion
During recruitment, leukocytes respond to specific inflammatory stimuli. The transition from cell rolling to arrest is initiated by the chemokine-triggered activation of GPCRs, leading to an upshift in LFA-1 affinity that facilitates firm binding to its ligand ICAM-1 on inflamed endothelium. Although this crucial initiation step is necessary for the efficient recruitment of leukocytes during inflammation, very little is known about the detailed contribution of the different GPCR subunits to LFA-1 activation. An earlier study by Zarbock et al7 demonstrated that Gαi2 signaling is crucially important for chemokine-induced arrest and leukocyte recruitment. In that study, Gβγ complex signaling might also be disturbed because the relevant α subunit was absent. Our experiments indeed revealed that each GNB isoform fulfills an indispensable role in GPCR-mediated downstream signaling leading to the activation of LFA-1. We demonstrated that downregulation of any single GNB isoform or GNAI2 leads to impaired IP3 generation, calcium flux, and subsequent LFA-1 activation. To demonstrate the physiological relevance of our finding, we showed that each Gnb isoform is required for effective neutrophil recruitment into the lung after LPS inhalation in a complex disease model and for chemokine-induced leukocyte arrest in vivo by using mixed chimeric mice with downregulated Gnb isoforms. We also elucidated the shared contribution of Plcβ isoforms in this signaling pathway. We showed the functional relevance of these findings with in vivo experiments and in a lung model of acute inflammatory insult.
The nonredundant function of these isoforms was contrary to expectation, because high similarities (homology) exist among GNB1, GNB2, and GNB4. It seemed reasonable to assume that each of the other isoforms would be able to replace the function of a downregulated isoform or that 1 or perhaps 2 isoforms are involved within the same pathway. Our findings were only partially in line with those of another study that used downregulated Gnb isoforms, which showed specialized roles for single isoforms.27 Previous in vitro experiments showed that murine Gnb2 is involved in determining the directionality of neutrophil chemotaxis, whereas Gnb1 knockdown impaired only bacteria-killing ability. Knocking down the expression of both Gnb1 and Gnb2 isoforms resulted in reduced phagocytosis.27 These differences might be the result of different experimental setups and the use of different stimuli. Zhang et al27 used formyl methionyl leucyl phenylalanine in their experiments, whereas we used CXCL1, suggesting that different chemokine receptors require different Gnb isoforms for downstream signaling. Gnb4 and Gnb5 were not investigated in this study. Noteworthy, several studies revealed that eliminating one isoform cannot be functionally substituted by other isoforms although they share high similarity at the amino acid level.28 Studies focused on the mechanisms of Gβγ signaling have revealed that in addition to the large variety of possible combinations of Gα, β, and γ subunits, cells can also establish compensatory mechanisms if one isoform is downregulated.29 However, nothing has been published on a common principle that may guide Gβγ signaling within various types of cells and via different stimuli. Apparently, this is the first study demonstrating a contribution of several Gnb isoforms to the same signaling pathway.
The nonredundant function of the isoforms was unforeseeable and raises the question of whether they act within 1 macromolecular complex on the same molecules or serve distinct roles in downstream signaling, even though each of them is indispensable for LFA-1 activation. The latter point is especially pertinent because Gβγ-mediated signaling elicits a large variety of downstream effectors such as PI3K and other PLC isoforms,28 which function in integrin activation and leukocyte recruitment. This apparently parallel contribution of Gnb isoforms to GPCR signaling that leads to LFA-1 activation seems complex and will require further studies to elucidate their specialized role.
Here, we demonstrate that chemokine (CXCL1 or IL-8) stimulation leads to the activation of both Plcβ2 and Plcβ3 in an Rac1-dependent manner as depicted in a model of putative downstream signaling after GPCR activation (Figure 6). This was quite unexpected, because previous studies concluded that Rac1 is located far downstream in this GPCR signaling pathway.20,30 In murine neutrophils, Rac1 has important functions downstream of calcium signaling in crawling and migrating cells.20 In T-lymphocytes, Rac1 belongs to a signaling module controlling LFA-1 activation that leads to lymphocyte arrest in high endothelial venules.30 The biology of Rac1 is very complex, and its spatial localization in the cell appears to be important for its function. In line with some in vitro studies, which convincingly demonstrate that Plcβ2 and Plcβ3 are able to bind to Rac1 via their PH domain,17 we show that the PH domain of Plcβ2 is capable of pulling down Rac1 in whole-cell lysates. Likewise, we report that the PH domains of Plcβ2 and Plcβ3 are required for integrin activation and leukocyte recruitment in vivo. Our data are consistent with that of a recent study using a mutant form of Plcβ2 (Plcβ2-Q52A), which revealed a reduced binding affinity to Rac1 and impaired polarized localization of the complex at the leading edge of chemotaxing cells.31
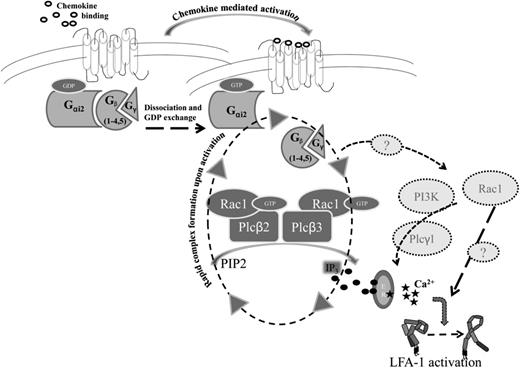
Schematic model of downstream signaling after GPCR activation. After chemokine binding, Gα and Gβγ subunits dissociate and GDP is exchanged for GTP. Gβ subunits, Rac1, Plcβ2, and Plcβ3 form a macromolecular complex upon activation, leading to generation of IP3 (black circles), which in turn binds to IP3-gated calcium stores leading to calcium release (black stars). Finally, activated LFA-1 upshifts to the high-affinity conformation. Dotted arrows, question marks, and molecules in light gray circles indicate potential alternative signaling pathways which might also play a role in downstream signaling leading to LFA-1 activation.
Schematic model of downstream signaling after GPCR activation. After chemokine binding, Gα and Gβγ subunits dissociate and GDP is exchanged for GTP. Gβ subunits, Rac1, Plcβ2, and Plcβ3 form a macromolecular complex upon activation, leading to generation of IP3 (black circles), which in turn binds to IP3-gated calcium stores leading to calcium release (black stars). Finally, activated LFA-1 upshifts to the high-affinity conformation. Dotted arrows, question marks, and molecules in light gray circles indicate potential alternative signaling pathways which might also play a role in downstream signaling leading to LFA-1 activation.
Plcβ2 and Plcβ3 fulfill an additive role in downstream signaling in that each isoform contributes to the generation of IP3 by hydrolysis of phosphatidylinositol 4,5-bisphosphate, which leads to a dose-dependent increase of intracellular calcium levels. Likewise, LFA-1 function is also dose dependent, and the full ICAM-1 binding ability and subsequent integrin clustering and stable neutrophil arrest are reliant on phospholipase activity of both Plcβ2 and Plcβ3. Alternatively, it is possible that Rac1 may also activate a yet undefined Ca2+-independent pathway that leads to integrin activation, which is independent of Plcβ isoforms. For instance, Rac1 might also be involved in the PI3K-mediated activation of PLCγ subunits that leads to IP3 generation and subsequent Ca2+ flux.32 Consistent with this are reports that PLCγ2 is not involved in chemokine-mediated arrest of neutrophils.33 Furthermore, PLCγ1 is expressed at much lower levels in hematopoietic cells,34 suggesting that it plays only a minor role within this context as depicted in Figure 6.
The physiological relevance of our findings was supported by data from the lung disease model, which revealed that phospholipase deficiency protected mice from LPS-induced lung injury. Although the defect in chemokine-induced arrest is more pronounced in Rac-1-deficient mice compared with Plcb2−/− and Plcb3−/− mice, all the knockout mice have the same number of neutrophils in the BAL, suggesting that Plcβ isoforms are also involved in other leukocyte recruitment steps.
Taken together, our data provide novel insight into the function of GPCR-mediated downstream signaling. We show for the first time, that the Gβγ complex is crucially involved in chemokine-induced LFA-1 activation and plays a role as important as that of Gαi2 signaling in the context of inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Brackebusch for providing the conditional Rac1-deficient mice.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (ZA428/6-1, ZA428/9-1, and SFB1009_A5) (A.Z.), Cells-in-Motion Cluster of Excellence EXC 1003-CiM from the University of Muenster, Muenster, Germany (A.Z.), and National Heart, Lung, and Blood Institute P01 HL078784 (K.L.) and National Institute of Allergy and Infectious Diseases R01 AI47294 (S.I.S.) from the National Institutes of Health.
Authorship
Contribution: H.B. and A.S. designed and performed most of the experiments, analyzed the results, and prepared the manuscript; D.R. and J.R. performed flow chamber and intravital microscopy experiments; C.S. performed biochemistry assays; G.G. performed in vitro experiments; D.W. provided the Plcb2−/− and Plcb3−/− mice and wrote part of the manuscript; and K.L. and A.Z. provided overall supervision, helped design all of the experiments, and were assisted by S.I.S. in preparing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Zarbock, University of Muenster, Department of Anesthesiology, Intensive Care and Pain Medicine, Albert-Schweitzer-Campus 1, Building A1, 48149 Muenster, Germany; e-mail: zarbock@uni-muenster.de.
References
Author notes
H.B. and A.S. contributed equally to this study and share first authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal