Key Points
BV and AVD followed by ISRT is well tolerated, without significant pulmonary toxicity.
BV and AVD followed by ISRT is an effective therapy for unfavorable-risk early stage HL, including bulky disease.
Abstract
This multicenter pilot study assessed the safety and efficacy of brentuximab vedotin (BV) and AVD (adriamycin, vinblastine, and dacarbazine) followed by 30 Gy involved site radiation therapy (ISRT). Patients with newly diagnosed, early stage classical Hodgkin lymphoma (HL) with unfavorable-risk features were treated with 4 cycles of BV and AVD. Patients who achieved a negative positron emission tomography (PET) scan (Deauville score of 1-3) received 30 Gy ISRT. Thirty patients received treatment and were assessable for toxicity. Twenty-nine patients completed 4 cycles of BV + AVD, and 25 patients BV + AVD + 30 Gy ISRT. No clinically significant noninfectious pneumonitis was observed. Serious adverse events (≥grade 3) were reported in 4 patients, including febrile neutropenia, peripheral neuropathy, and hypertension. After 2 and 4 cycles of BV + AVD, 90% (26 of 29) and 93% (27 or 29) of patients achieved a negative PET scan, respectively. Two patients with biopsy-proven primary refractory HL were treated off-study. All 25 patients who completed BV + AVD + ISRT achieved a complete response. With a median follow-up of 18.8 months, by intent to treat, the 1-year progression-free survival is 93.3% (95% confidence interval, 84-102). Overall, the treatment was well-tolerated with no evidence of significant pulmonary toxicity. The majority of patients (≥90%) achieved negative interim PET scans after 2 and 4 cycles of BV + AVD. Excluding the 2 primary refractory patients, all patients are disease free, suggesting that this is a highly active treatment program even in patients with substantial disease bulk. This trial was registered at www.clinicaltrials.gov as #NCT01868451.
Introduction
The standard of care for patients with early stage, unfavorable-risk Hodgkin lymphoma (HL), particularly patients with disease bulk, is chemotherapy in combination with radiation therapy (RT).1 Although recent studies have demonstrated efficacy for chemotherapy-alone approaches in selected early stage, unfavorable-risk patients based on favorable interim positron emission tomography (PET) response, these studies have generally excluded patients with bulky disease or systemic symptoms.2,3 The estimated 5-year progression-free survival (PFS) for patients with early stage, unfavorable-risk disease treated with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) and involved-field radiotherapy is 80% to 85%; thus a significant proportion of patients will relapse in this population.4-6
Because of an increased appreciation of long-term toxicities related to RT in HL survivors who receive combined modality therapy (CMT), efforts have been made to reduce the doses and the extent of RT fields over time.7,8 Involved-nodal RT, applied in the European Organisation for Research and Treatment of Cancer Lymphoma Group / Lymphoma Study Association / Fondazione Italiana Linfomi (EORTC/LYSA/FIL) H10 study, involves a markedly reduced RT field compared with its predecessor involved-field RT (IFRT) and encompasses only the prechemotherapy disease volume with minimal margins.3,9 Conceptually similar to involved-nodal RT, but with less strict pretreatment imaging requirements, involved-site radiotherapy (ISRT) is now the standard RT field for HL patients as recommended by the International Lymphoma Radiation Oncology Group and the National Comprehensive Cancer Network guidelines.10
Brentuximab vedotin (BV; Seattle Genetics; Bothell, WA) is a novel antibody-drug conjugate that is highly active in the treatment of patients with relapsed and refractory HL.11-13 In a phase 1 study in the frontline setting, patients with advanced stage HL were treated with BV at a dose of 1.2 mg/kg in combination with AVD chemotherapy for up to 6 cycles.14,15 For the 26 patients treated with BV + AVD, the treatment was found to be safe, and the preliminary efficacy was promising with a 3-year failure-free survival and overall survival of 96% and 100%, respectively.14,15 With the aim of improving initial cure rates, many ongoing studies are testing the combination of BV with AVD chemotherapy for the frontline treatment of HL in various clinical settings.
Limited data exist on the administration and safety of BV in conjunction with CMT for the frontline treatment of early stage HL. Pulmonary toxicity is a known of side effect of bleomycin and radiation involving the mediastinum and lung fields.16-19 In addition, combining BV with the bleomycin-containing ABVD arm in the phase 1 study resulted in significant pulmonary toxicity, and, analogously, when SGN-30 (naked anti-CD30 monoclonal antibody) was combined with gemcitabine, severe grade 3 to 5 pneumonitis led to premature study closure.14,20 We hypothesized that BV may be associated with subclinical pulmonary toxicity that is only unmasked when combined with an additional pulmonary insult, such as bleomycin or radiotherapy.
We designed this pilot study to assess the safety and preliminary efficacy of the up-front combination of BV with AVD chemotherapy followed by 30-Gy ISRT for patients with early stage, unfavorable-risk HL. The primary objective of this study was to evaluate the rate of development of clinically significant pulmonary toxicity with the proposed treatment program. The secondary objective was to evaluate the preliminary efficacy.
Methods
Study population
Patients between the ages of 18 and 60 with biopsy-proven CD30-positive classical HL were enrolled. Eligible patients had untreated stage I/II, classical HL with any of the unfavorable-risk factors as defined by German Hodgkin Study Group (GHSG) including bulky mediastinal mass (≥1/3 maximum width of mass/maximum intrathoracic diameter (mediastinal mass ratio [MMR]) on posterioranterior chest X-ray (CXR) or ≥10 cm by computed tomography [CT] imaging in transaxial plane), erythrocyte sedimentation rate (ESR) ≥50 mm/h or ESR ≥30 mm/h in patients with “B” symptoms, extranodal involvement, or >2 lymph node sites (as defined by GHSG).5 In addition, patients with infradiaphragmatic disease were eligible. Stage IIB disease with disease bulk (X) or extranodal involvement (E) were included. Patients had to have adequate organ function (defined as a cardiac ejection fraction of >50%, hemoglobin-adjusted diffusing capacity for carbon monoxide [DLCO(Hb)] ≥60% on pulmonary function testing [PFT], a serum creatinine clearance ≥30 mL/min, an absolute neutrophil count of >1000/µL, a platelet count >75 000/µL, and total bilirubin of <2.0 mg/dL in the absence of a history of Gilbert disease). Patients were excluded if pregnant or lactating, with baseline peripheral neuropathy >grade 1, or receiving chronic steroid treatment. Patients were enrolled on study from June 2013 to February 2015.
This pilot study was conducted at Memorial Sloan Kettering Cancer Center (MSKCC) and Wilmot Cancer Institute, University of Rochester (www.clinicaltrials.gov, #NCT01868451). The institutional review board of each participating institution approved the study, and written informed consent was obtained for all patients before enrollment.
Study design and treatment
Patients were scheduled to receive a total of 4 cycles of BV and AVD chemotherapy. BV (1.2 mg/kg), doxorubicin (25 mg/m2), vinblastine (6 mg/m2), and dacarbazine (375 mg/m2) were administered on days 1 and 15 of each 28-day cycle. Prophylactic growth factor support (Neupogen) was administered with each cycle, dosing and schedule per physician discretion.
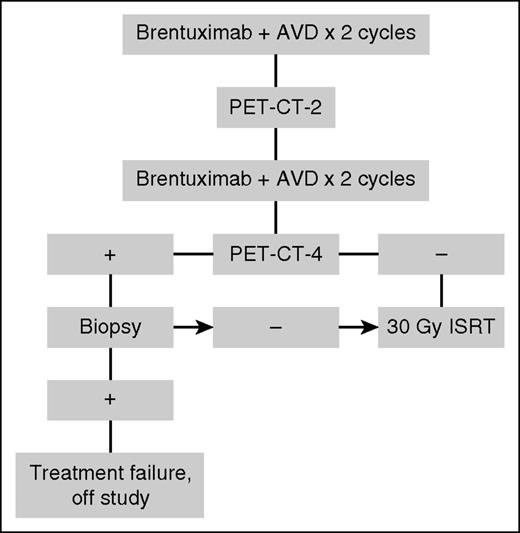
Patients with a PET-negative response after 4 cycles of BV and AVD chemotherapy or PET-positive postchemotherapy with subsequent biopsy negative for HL received 30-Gy ISRT. Patients with a positive PET-4 result and biopsy positive for lymphoma were subsequently treated off study. The treatment schema is summarized in Figure 1.
ISRT was administered per standard guidelines and under the direction of lymphoma radiation oncologists (J.Y. and L.S.C.).21 Typical ISRT fields were designed with consideration of the prechemotherapy and postchemotherapy gross tumor volume information. The resulting clinical target volume was then reduced so that normal structures such as lungs and heart, which were previously displaced by the gross tumor volume, were excluded. The clinical target volume was expanded 0.5 to 1.0 cm to planning target volume to account for setup error.10,21
Study assessments
All patients underwent standard staging tests including fluorodeoxyglucose-PET; diagnostic CT scans of neck (if evidence of disease in neck), chest, abdomen, and pelvis; PA and lateral chest radiograph; and unilateral bone marrow biopsy if presence of B symptoms, extranodal disease, or mass >10 cm.
PET scan was performed after 2 cycles and 4 cycles of BV + AVD, and again 8 weeks after completion of BV + AVD + ISRT. All PET scans were reviewed by nuclear medicine radiologists at MSKCC and Wilmot Cancer Institute and interpreted using the 5-point Deauville scale; a score of 1, 2, or 3 was negative.22 The interim PET after 2 cycles was exploratory; treatment was not altered based on the result.
Safety assessments consisted of recordings of adverse events (AEs), physical examination, PFTs, and routine laboratory tests. AEs were assessed per the Common Terminology Criteria of Adverse Events (CTCAE v4.03). PFTs were obtained at baseline, post-(BV + AVD), post-(BV + AVD + ISRT), and 12 months posttreatment. PFTs were performed in pulmonary laboratories, and percent predicted DLCO(Hb) and forced vital capacity (FVC) were determined using the same references equations across all PFT studies.23,24
Outcomes
The central study aim was to assess the safety and toxicities of the treatment regimen. The primary objective was to evaluate the rate of the development of significant pulmonary toxicity, which was defined as the development of grade 2 or higher noninfectious pneumonitis as defined by CTCAE v4.03. All patients with suspected pulmonary toxicity were evaluated by an institutional pulmonary specialist who determined if the patient’s clinical picture was consistent with noninfectious pneumonitis and adjudicated the grade.
The secondary objective was to evaluate the preliminary efficacy of the proposed treatment plan, including response to therapy and 1-year PFS.
Statistical analyses
Descriptive statistics were used to describe toxicities and response rates. The trial included a stopping rule to declare unacceptable toxicity rates; if >1 out of the first 15 patients or >3 out of the total 30 patients developed significant pulmonary toxicity, the trial would have been stopped. PFS was estimated using the Kaplan-Meier method. The study database was frozen in February 18, 2016.
Results
Patients
From June 2013 to February 2015, 30 patients were enrolled. The median age of enrolled patients was 31 years (range, 18-59) and all patients had stage II disease. With regard to unfavorable-risk features, 47% of patients had disease bulk by CT criteria >10 cm, 57% had disease bulk by ≥1/3 MMR on CXR, 67% had elevated ESR (ESR >50 or ESR >30 with B symptoms), 47% presented with B symptoms, 47% had extranodal involvement, 67% had >2 involved lymph node sites, and 3% presented with infradiaphragmatic disease. Thirteen patients had a bulky anterior mediastinal mass with a median maximal transverse diameter of 13 cm, range 10 to 16.9 cm. Applying a novel MSKCC definition of disease bulk in early stage HL (>7 cm in maximal transverse diameter or >7 cm in maximal coronal diameter), 23 (77%) patients had disease bulk.25 Eleven patients (37%) had advanced stage disease by the GHSG criteria: 2 (7%) with stage IIBX, 4 (13%) with stage IIBE, and 5 (17%) with stage IIBXE disease. Baseline patient characteristics are shown in Table 1.
Patient characteristics of early stage HL, N = 30
| Characteristic . | Patients . | |
|---|---|---|
| No. . | % . | |
| Age, median [range], y | 30 | 31 [18-59] |
| Sex | ||
| Male | 16 | 53 |
| Female | 14 | 47 |
| HL pathologic features | ||
| CD20+ | 4 | 13 |
| EBV+ (by EBER-ISH or LMP1), N = 27 | 3 | 11 |
| Stage II | 30 | 100 |
| Unfavorable risk features | ||
| B symptoms | 14 | 47 |
| ESR >50 or ESR >30 with B symptoms | 20 | 67 |
| Nodal sites >2 | 20 | 67 |
| Extranodal involvement | 14 | 47 |
| Bulk ≥10 cm on CT | 14 | 47 |
| Bulk ≥1/3 MMR on CXR | 17 | 57 |
| Anterior mediastinal mass >10 cm, N = 14, median size in cm [range] | 13 | [10-16.9] |
| Bulky by MSK definition (>7 cm in MTD or >7 cm in MCD), N = 30 | 23 | 77 |
| Advanced stage by GHSG criteria | 11 | 37 |
| IIBX | 2 | 7 |
| IIBE | 4 | 13 |
| IIBXE | 5 | 17 |
| Characteristic . | Patients . | |
|---|---|---|
| No. . | % . | |
| Age, median [range], y | 30 | 31 [18-59] |
| Sex | ||
| Male | 16 | 53 |
| Female | 14 | 47 |
| HL pathologic features | ||
| CD20+ | 4 | 13 |
| EBV+ (by EBER-ISH or LMP1), N = 27 | 3 | 11 |
| Stage II | 30 | 100 |
| Unfavorable risk features | ||
| B symptoms | 14 | 47 |
| ESR >50 or ESR >30 with B symptoms | 20 | 67 |
| Nodal sites >2 | 20 | 67 |
| Extranodal involvement | 14 | 47 |
| Bulk ≥10 cm on CT | 14 | 47 |
| Bulk ≥1/3 MMR on CXR | 17 | 57 |
| Anterior mediastinal mass >10 cm, N = 14, median size in cm [range] | 13 | [10-16.9] |
| Bulky by MSK definition (>7 cm in MTD or >7 cm in MCD), N = 30 | 23 | 77 |
| Advanced stage by GHSG criteria | 11 | 37 |
| IIBX | 2 | 7 |
| IIBE | 4 | 13 |
| IIBXE | 5 | 17 |
EBV, Epstein-Barr virus; EBER-ISH, Epstein-Barr encoding region in situ hybridization; LMP1, latent membrane protein 1; MCD, maximal coronal diameter; MSK, Memorial Sloan Kettering; MTD, maximal transverse diameter.
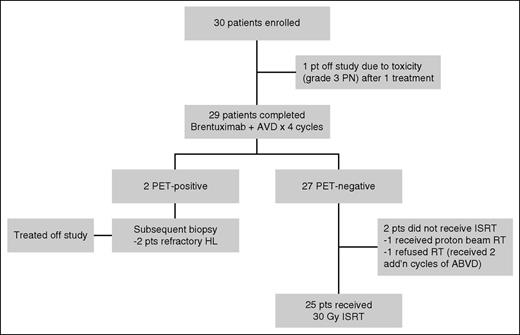
A flowchart of the study is shown in Figure 2. Of 30 patients, 29 were treated with 4 cycles of BV and AVD chemotherapy. One patient came off study because of toxicity after 1 treatment of BV + AVD. Four patients did not receive 30-Gy ISRT. Of these, 2 patients had biopsy-confirmed refractory classical HL, 1 patient elected to come off study and received proton beam RT to the mantle field, and 1 patient elected to come off study and received chemotherapy alone with 2 additional cycles of ABVD after completing 4 cycles of BV + AVD therapy.
After completing 4 cycles of BV + AVD, 25 patients received ISRT at a dose of 30.6 Gy in 17 daily fractions. Sixty percent of patients (n = 15) were simulated and treated using deep inspiration breath hold to maximally spare normal lung and heart tissue. Ninety-six percent (n = 24) of patients received radiation to the neck and mediastinum, and the median percentage of lung volume receiving 20-Gy radiation was 18.5% (range 3% to 36%; supplemental Figure 1, available on the Blood Web site). A single patient received conventional radiation to the left pelvis. Forty-four percent of patients (n = 11) received intensity-modulated RT for extensive bilateral local disease involving the neck and chest. A remaining 13 patients (52%) received conventional RT using anterior-posterior–posterior-anterior fields and a single patient received three-dimensional–conformal RT.
Safety and toxicity
AEs associated with BV + AVD were generally grade 1 or 2 and easily managed, and the most common grade 3 or higher AE was the development of neutropenia (16 [53%] of 30 patients). As anticipated with the combination of BV and vinblastine, peripheral neuropathy was observed in 40% of patients (12 of 30 patients), most grade 1 (10 [83%] of 12 patients) and less frequent grade 3 (2 [17%] of 12 patients). Serious AEs occurred in 6 patients. Of these serious AEs, 3 patients had febrile neutropenia (grade 3), and 2 patients were admitted with fever without neutropenia (grade 1/2). One patient, after a single treatment of BV + AVD, developed hypertension (grade 3) and peripheral motor and sensory neuropathy (grade 3) and came off study.
All patients tolerated ISRT well with only grade 1 to 2 toxicity. The most common acute toxicities were fatigue, which affected 88% of patients (n = 22), followed by dermatitis (n = 20), dysphagia (n = 20), and esophagitis (n = 13). Of the 24 patients who received ISRT to the mediastinum, 9 patients (38%) reported grade 1 cough, and a single patient (4%) reported grade 1 dyspnea.
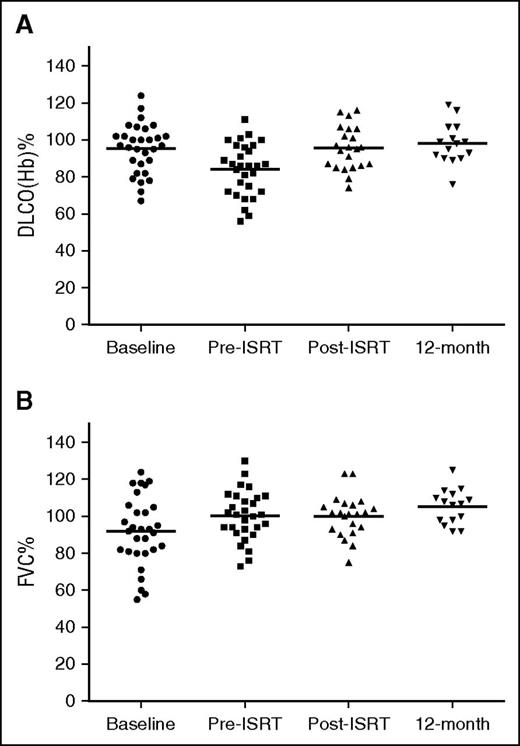
No clinically significant (grade 2 or higher) drug-related noninfectious pneumonitis was observed. In most patients, there was an initial decline in DLCO(Hb) after 4 cycles of BV + AVD. There was no change in FVC related to treatment with BV (Figure 3B). Administration of ISRT was not associated with a worsening of the DLCO(Hb). Changes in DLCO(Hb) were not accompanied by respiratory symptoms in most patients. Two patients were evaluated by a pulmonary specialist. One patient was evaluated because of cough and dyspnea (grade 2) that developed after completion of 4 cycles of BV and AVD chemotherapy. PFTs showed an initial decrease by 30% in DLCO(Hb) from baseline, but repeat PFTs 1 week later showed an increase by 16%. The respiratory symptoms were attributed to an upper respiratory infection and reactive airways disease, not drug related, and resolved with antibiotics and supportive care. Of note, this patient had normal repeat PFTs 12 months posttreatment. The second patient evaluated by a pulmonary specialist had mild dyspnea on exertion (grade 1) and an initial decrease in DLCO(Hb) by 57% after chemotherapy compared with baseline; however, this was attributed to an upper respiratory infection and poor test effort, not related to BV + AVD. DLCO(Hb) improved on repeat PFTs.
Trend in DLCO(Hb) and FVC during the treatment program. (A) Mean DLCO(Hb) at baseline, pre-ISRT, post-ISRT, and 12 months posttreatment are 91% (n = 30), 82% (n = 29), 92% (n = 23), and 98% (n = 15), respectively. (B) Trend in FVC during treatment program. Mean FVC at baseline, pre-ISRT, post-ISRT, and 12 months posttreatment are 91% (n = 30), 97% (n = 28), 99% (n = 22), and 106% (n = 15), respectively.
Trend in DLCO(Hb) and FVC during the treatment program. (A) Mean DLCO(Hb) at baseline, pre-ISRT, post-ISRT, and 12 months posttreatment are 91% (n = 30), 82% (n = 29), 92% (n = 23), and 98% (n = 15), respectively. (B) Trend in FVC during treatment program. Mean FVC at baseline, pre-ISRT, post-ISRT, and 12 months posttreatment are 91% (n = 30), 97% (n = 28), 99% (n = 22), and 106% (n = 15), respectively.
Interim PET response
Ninety percent of patients (26 of 29) achieved a negative PET scan after 2 cycles (Table 2). Of patients with disease bulk treated with 2 cycles of BV and AVD chemotherapy, 85% (11 of 13) were PET-2 negative. Of the 3 patients with PET-2 positive scans, the scan became negative after 4 cycles in 2 patients, and 1 patient had biopsy-proven primary refractory HL. Ninety-three percent of patients (27 of 29) had a negative PET scan after 4 cycles of chemotherapy. The 2 patients with a positive PET scan after 4 cycles had baseline tumor bulk (measuring 13.0 and 13.6 cm in maximal transverse diameter on CT). Both had a positive biopsy consistent with refractory HL and were treated off study with ifosfamide, carboplatin, and etoposide chemotherapy, pretransplantation radiation, and high-dose therapy and autologous stem cell transplant. These patients continue in remission 7 and 13 months posttransplant.
PET results after 2 and 4 cycles of BV + AVD chemotherapy and at the end of treatment (EOT)
| Deauville score . | Post 2 cycles (n = 29) . | Post 4 cycles (n = 29) . | EOT/post-ISRT (n = 25) . |
|---|---|---|---|
| 1* | 3 (10%) | 5 (20%) | |
| 2* | 14 (48%) | 21 (72%) | 16 (64%) |
| 3* | 12 (41%) | 3 (10%) | 2 (8%) |
| 4 | 3 (10%) | 2 (7%) | 2 (8%)† |
| 5 |
| Deauville score . | Post 2 cycles (n = 29) . | Post 4 cycles (n = 29) . | EOT/post-ISRT (n = 25) . |
|---|---|---|---|
| 1* | 3 (10%) | 5 (20%) | |
| 2* | 14 (48%) | 21 (72%) | 16 (64%) |
| 3* | 12 (41%) | 3 (10%) | 2 (8%) |
| 4 | 3 (10%) | 2 (7%) | 2 (8%)† |
| 5 |
EOT indicates 8 wk after completion of ISRT.
PET-negative response was defined as Deauville score 1 to 3.
For 1 patient, repeat PET scan remained positive, but subsequent biopsy demonstrated granulomatous inflammation consistent with sarcoid. For the second patient, repeat PET after 2 mo was negative (Deauville 3). Thus, both patients achieved a complete response.
End of treatment response
All patients who completed the entire treatment program with BV + AVD followed by ISRT (n = 25) achieved a complete response. Two patients had a positive PET posttreatment (Deauville 4; Table 2). For 1 patient, repeat PET scan remained positive, but subsequent biopsy demonstrated granulomatous inflammation consistent with sarcoid. For the second patient, a repeat PET scan after 2 months was negative (Deauville 3). Thus, both patients achieved a complete response.
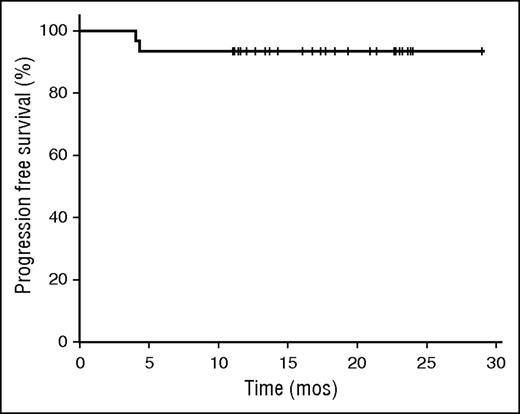
The median follow-up time is 18.8 months. Of the 25 patients who have completed CMT and achieved complete responses, no relapses have occurred to date. By intent to treat including all 30 patients, the 1-year PFS is 93.3% (95% confidence interval [CI], 84-102; Figure 4).
Discussion
To our knowledge, this is the first publication describing the safety and tolerability of BV in combination with chemoradiotherapy for the treatment of early stage HL. Overall the treatment program was well tolerated and associated with preliminary evidence of promising efficacy with high rates of early PET negativity and excellent 1-year PFS.
The study was designed to primarily assess if there was evidence of undue toxicity associated with BV and CMT, with particular attention to pulmonary toxicity given the incidence of fatal lung injury with the combination of BV and bleomycin.14 In this study, no significant noninfectious pneumonitis occurred with the combination of BV and chemoradiotherapy. Pulmonary function studies demonstrated a decrement in DLCO in majority of patients after 4 cycles of BV and AVD chemotherapy. We hypothesize that this decrease is DLCO can be attributed to brentuximab-mediated subclinical mild alveolar injury as AVD is not associated with pulmonary toxicity and underlying intrathoracic disease burden was significantly reduced after chemotherapy. Further, there was evidence of interval improvement in FVC after BV + AVD, which demonstrates an absence of significant drug-related restrictive lung injury and likely reflects reduction in mediastinal disease burden after treatment. In addition, no clinically significant drug-related pulmonary toxicity was observed. For the 2 patients referred to a pulmonologist, their respiratory symptoms and transient PFT abnormalities were attributed to upper respiratory infections. Similarly, in the phase 1 experience of BV + AVD, no patients experienced pulmonary toxic effects.14 In the current study, despite the fact that most patients received RT to the mediastinum, radiotherapy did not worsen lung diffusing capacity or FVC or give rise to clinically significant respiratory symptoms. In addition, 12-month PFTs showed improvement in mean DLCO(Hb) and FVC even higher than baseline likely because of resolution of intrathoracic HL. This compares favorably with retrospective data for ABVD and mantle RT, which demonstrated decrement in DLCO and FVC at 12 to 15 months posttreatment.17,18
Although interim PET negativity is an important prognostic factor in early stage HL, a robust historical benchmark to compare our interim PET-2 results does not exist given the heterogeneity of early stage HL patient populations and variable definitions for disease bulk and PET negativity across studies.3,26 The EORTC/LYSA/FIL H10 is the only prospective study with interim PET-2 data, and ∼76% of early stage, unfavorable-risk patients (40% with EORTC defined disease bulk) had a negative PET scan after 2 cycles of ABVD applying the now outdated International Harmonization Project Criteria (PET negative = Deauville score 1 or 2).3 In one of the largest retrospective series of interim PET in early stage HL, 85% of patients (67/79) with early stage, unfavorable-risk HL with EORTC-defined disease bulk achieved a negative PET (Deauville 1-3) after 2 cycles of ABVD.27 In our pilot study including early stage, unfavorable-risk HL patients with 47% of patients meeting GHSG disease bulk criteria, 90% of patients achieved a negative PET (Deauville 1-3) after 2 cycles of BV + AVD. In the phase 1 study of BV + AVD in advanced stage HL, a similar PET-negative rate (24 of 26 patients, 92%) was demonstrated.14 Preliminarily BV and AVD compares favorably to standard ABVD in terms of early conversion to PET negativity after 2 cycles, suggesting it is a highly active treatment program in this group of early stage HL patients with substantial tumor bulk and extensive disease. Overall the reduced rate of PET positivity with BV + AVD therapy is encouraging as interim-PET positivity has been associated with inferior outcome in HL and in risk-adapted protocols prompts further intensification of therapy.28
This study was not designed to definitively assess the efficacy of BV + AVD × 4 cycles followed by 30-Gy ISRT, and we cannot formally compare our results to previous trials because of differences in eligibility, type and number of cycles of chemotherapy, and radiotherapy field and dose. Nevertheless, the 1-year PFS outcome for the current treatment program, 93% (95% CI, 84-100), appears promising and is approximately equivalent to previously reported data for combined modality therapy programs. For example, in the Intergroup E2496 trial that included early stage HL patients with locally extensive, bulky mediastinal disease, among patients treated with ABVD × 6 to 8 cycles + 36-Gy IFRT, the 1-year PFS was 94% (95% CI, 90-98) (Ranjana Advani, Stanford University Medical Center, e-mail, January 19, 2016).6 Compared with the E2496 trial, our treatment program uses fewer cycles of chemotherapy (4 vs 6-8) and a smaller radiotherapy dose and field (30-Gy ISRT vs 36-Gy IFRT). Based on data from the GHSG HD11 and HD14 clinical trials, the 1-year PFS for the ABVD × 4 cycles + 30-Gy IFRT arms was 96.6% (95% CI, 94.7-98.5) and 96.1% (95% CI, 94.7-97.5), respectively (Andreas Engert, University Hospital of Cologne, e-mail, April 30, 2016; Bastian von Tresckow, University Hospital of Cologne, e-mail, April 26, 2016).5,29 However, the patients included in the GHSG trials were more favorable as they did not include patients with stage IIBX, IIBE, or IIBXE disease (classified as advanced stage by GHSG criteria), and in our study, 37% of patients met these criteria.
A limitation of this study is the small sample size that was adequate for assessing toxicity, but inadequate for a robust efficacy assessment. To further explore efficacy, we have expanded the current study to include an additional 29 patients who will receive a decreased dose of ISRT, 20 Gy. In addition, the median follow-up time in this study is only 18.8 months, although our data fully capture the rate of primary refractory disease (< complete response), which is a significant concern among patients with bulky HL.
In conclusion, BV in conjunction with AVD chemotherapy and ISRT is a well-tolerated regimen with no evidence of pulmonary toxicity. The early results from this study demonstrate promising efficacy with high rates of PET negativity and complete responses. Recent data from the British Columbia Cancer Agency report excellent outcomes for HL patients with disease bulk who achieve a negative PET scan after full-course ABVD without additional consolidative RT.30 Future studies to test whether radiotherapy can be eliminated or its volume further reduced in PET-negative bulky early stage HL patients treated with BV and AVD chemotherapy are warranted.
Presented at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 7, 2014; and at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 18, 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the participating patients and their families. The authors thank Seattle Genetics for providing BV for this study. The authors also thank the GHSG and Eastern Cooperative Oncology Group investigators for providing 1-year PFS data for prior combined modality therapy clinical trials in early stage HL.
Financial support for this research was received from the Lymphoma Research Foundation, Seattle Genetics, and the National Institutes of Health, National Cancer Institute Core Grant P30 CA008748.
Authorship
Contribution: A.K., C.C., J. Yahalom, H.S., P.M.B., P.C., A.C., L.S.C., P.D., J.W.F., J.F.G., A.H., P.A.H., S.M.H., A.G.J., M.J.M., G.N.M., S.J.M., A.J.M., A.N., M.L.P., C.S.P., D.J.S., N.V., S.L.V., J. Yang, A.Y., A.D.Z., Z.Z., and C.H.M. contributed to the study design, patient recruitment, data collection, and data analysis; A.K. and C.H.M. wrote the manuscript; and all authors commented on and revised the manuscript.
Conflict-of-interest disclosure: P.D., S.J.M., and A.J.M. served on an advisory board for Seattle Genetics and received an honorarium. A.Y. received an honorarium from Seattle Genetics and Takeda Millennium. C.H.M. and P.M.B. have consulted for Seattle Genetics. C.H.M. serves on the scientific advisory board for Seattle Genetics. A.K., A.J.M., and C.H.M. receive research funding from Seattle Genetics. J.W.F. has received an honorarium from Bayer for the Data Safety Monitoring Board. The remaining authors declare no competing interests.
Correspondence: Anita Kumar, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 468, New York, NY 10065; e-mail: kumara2@mskcc.org.