Key Points
Anti-BCMA T cells have impressive activity against MM.
Abstract
Therapies with novel mechanisms of action are needed for multiple myeloma (MM). B-cell maturation antigen (BCMA) is expressed in most cases of MM. We conducted the first-in-humans clinical trial of chimeric antigen receptor (CAR) T cells targeting BCMA. T cells expressing the CAR used in this work (CAR-BCMA) specifically recognized BCMA-expressing cells. Twelve patients received CAR-BCMA T cells in this dose-escalation trial. Among the 6 patients treated on the lowest 2 dose levels, limited antimyeloma activity and mild toxicity occurred. On the third dose level, 1 patient obtained a very good partial remission. Two patients were treated on the fourth dose level of 9 × 106 CAR+ T cells/kg body weight. Before treatment, the first patient on the fourth dose level had chemotherapy-resistant MM, making up 90% of bone marrow cells. After treatment, bone marrow plasma cells became undetectable by flow cytometry, and the patient’s MM entered a stringent complete remission that lasted for 17 weeks before relapse. The second patient on the fourth dose level had chemotherapy-resistant MM making up 80% of bone marrow cells before treatment. Twenty-eight weeks after this patient received CAR-BCMA T cells, bone marrow plasma cells were undetectable by flow cytometry, and the serum monoclonal protein had decreased by >95%. This patient is in an ongoing very good partial remission. Both patients treated on the fourth dose level had toxicity consistent with cytokine-release syndrome including fever, hypotension, and dyspnea. Both patients had prolonged cytopenias. Our findings demonstrate antimyeloma activity of CAR-BCMA T cells. This trial was registered to www.clinicaltrials.gov as #NCT02215967.
Introduction
Multiple myeloma (MM) is an almost always incurable malignancy of plasma cells.1,2 Although many therapies are available for MM,1-3 novel therapies that act by different mechanisms of action than current therapies are clearly needed.
Chimeric antigen receptors (CARs) are proteins that incorporate an antigen recognition domain, costimulatory domains, and T-cell activation domains.4-8 T cells genetically modified to express CARs specifically recognize and eliminate malignant cells expressing a targeted antigen.6,9-13 CAR-expressing T cells targeting the B-cell antigen CD19 can induce lasting complete remissions of B-cell malignancies.14-23 Toxicities that are mainly caused by cytokines (cytokine-release syndrome [CRS]) also occur after CAR T-cell infusions.14,24,25 The effectiveness of anti-CD19 CAR T cells against B-cell malignancies encouraged us to develop a CAR T-cell therapy for multiple myeloma.
Appropriate target antigens for CAR T-cell therapies should be uniformly expressed on the malignancy to be treated and should not be expressed on normal essential cells.5,26 We targeted B-cell maturation antigen (BCMA).27,28 BCMA is a member of the tumor necrosis factor superfamily.29 Among hematologic cells, BCMA is only expressed by some B cells, normal plasma cells, and malignant plasma cells; BCMA is not expressed by hematopoietic stem cells.12,30-32 We have shown after extensive polymerase chain reaction (PCR) and immunohistochemistry (IHC) experiments that BCMA is uniformly expressed by the malignant plasma cells of many cases of MM and that BCMA is not expressed by normal essential nonhematopoietic tissues.12 We designed the first anti-BCMA CAR,12 and now we have conducted the first-in-humans clinical trial of anti–BCMA-CAR T cells.
Materials and methods
Trial design
This phase 1 dose-escalation trial was approved by the National Cancer Institute Institutional Review Board. All patients provided informed consent. An Investigational New Drug Application for anti-BCMA CAR T cells was evaluated and permitted by the US Food and Drug Administration.
The goals of the trial were to assess the safety of anti-BCMA CAR T cells and to assess for early indications of antimyeloma activity. Eligibility criteria included essentially normal major organ function and measurable MM. We only enrolled patients with MM with uniform BCMA expression by either IHC or flow cytometry, meaning that no clear BCMA-negative populations of plasma cells were detected. Flow cytometry was generally more sensitive than IHC at detecting BCMA, and all treated MMs had uniform BCMA expression by flow cytometry.
All patients received 3 doses of 300 mg/m2 cyclophosphamide and 3 doses of 30 mg/m2 fludarabine. Chemotherapy was administered because experience in mice has demonstrated that recipient leukocyte depletion enhances the activity of adoptively transferred T cells.33-35 Both chemotherapy agents were administered daily on days −5, −4, and −3 before CAR-BCMA T-cell infusion on day 0. A single dose of CAR-BCMA T cells was administered to each patient. The dose escalation plan called for an initial dose of 0.3 × 106 CAR+ T cells/kg with threefold increases to each subsequent dose level. Progression to the next highest dose level was allowed after 3 patients were treated on a dose level without a dose-limiting toxicity. Data from all treated patients are included in this report. One patient was enrolled but did not receive any protocol treatment due to rapid clinical deterioration caused by myeloma progression; this patient was not included in this report.
Follow-up and staging
Myeloma staging was conducted according to the International Uniform Response Criteria for Multiple Myeloma.36 Toxicity was graded by the Common Terminology Criteria for Adverse Events version 4.02. Two weeks, 1 month, 2 months, 3 months, and 6 months after CAR-BCMA infusion, MM was assessed with standard staging tests.36
CAR T-cell production
Autologous peripheral blood mononuclear cells (PBMCs) were cultured with an anti-CD3 monoclonal antibody to induce T-cell proliferation. The cells were transduced with the γ-retroviral vector that encoded that CAR, and 9 days after the initiation of cultures, CAR-BCMA T cells were infused. Details of the CAR T-cell production are in supplemental Methods, available on the Blood Web site.
Immunologic assays
CAR-BCMA T cells were detected by flow cytometry after staining with a phycoerythrin-labeled BCMA constant-fragment reagent (PE-BCMA-Fc) (Figure 1B-C). CAR-BCMA T cells were also detected by performing quantitative PCR (qPCR). Enzyme-linked immunosorbent assays (ELISA) for interferon γ (IFNγ) were performed on supernatants from cultures of CAR-BCMA T-cell samples plus target cells. Interleukin-6 (IL-6) ELISAs, soluble BCMA ELISAs, and multicytokine assays were performed on patient serum. Methods for all assays are in supplemental Methods.
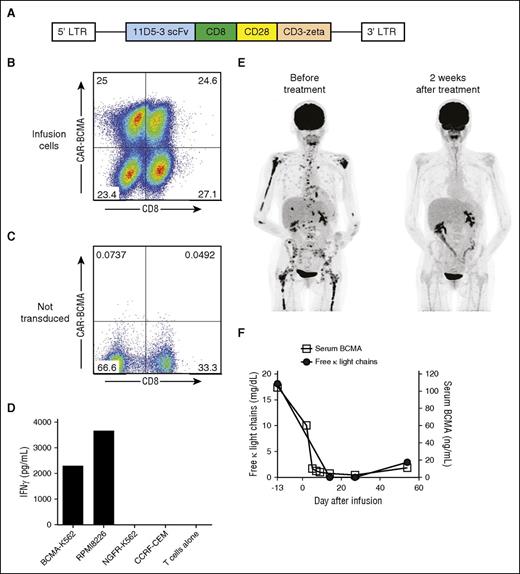
CAR-BCMA T cells specifically recognized BCMA in vitro and exhibited antimyeloma activity in humans. (A) Diagram of the MSGV-11D5-3-CD828Z γ-retroviral vector encoding the anti-BCMA CAR (CAR-BCMA) is shown. CAR-BCMA contained the 11D5-3 anti-BCMA single chain variable fragment (scFv), CD8α hinge and transmembrane regions, the cytoplasmic portion of the CD28 costimulatory moiety, and the CD3ζ T-cell activation domain. (B) CAR-BCMA expression on the surface of the infusion T cells of patient 10 was detected by staining with a PE-BCMA-Fc protein reagent. The plot is gated on live CD3+ lymphocytes. (C) Demonstrating specificity, the PE-BCMA-Fc reagent did not stain PBMCs that were not transduced with the CAR-BCMA gene. (D) CAR-BCMA T cells of patient 10 were cultured with target cells that either expressed BCMA (BCMA-K562 and RPMI8226) or did not express BCMA (NGFR-K562 and CCRF-CEM). The CAR-BCMA T cells of patient 10 specifically released IFNγ in response to BCMA-expressing target cells in vitro. (E) Patient 8 who had MM that was progressing despite 8 prior lines of therapy obtained a very good partial remission (VGPR) after infusion of CAR-BCMA T cells. Positron emission tomography–computed tomography scans from before and after treatment show elimination of a large number of MM bone lesions. (F) Patient 8’s free κ light chains level decreased after CAR T-cell infusion as the MM entered a VGPR and then increased with progression of the MM. The serum BCMA protein level followed a pattern similar to that of the serum free κ light chains.
CAR-BCMA T cells specifically recognized BCMA in vitro and exhibited antimyeloma activity in humans. (A) Diagram of the MSGV-11D5-3-CD828Z γ-retroviral vector encoding the anti-BCMA CAR (CAR-BCMA) is shown. CAR-BCMA contained the 11D5-3 anti-BCMA single chain variable fragment (scFv), CD8α hinge and transmembrane regions, the cytoplasmic portion of the CD28 costimulatory moiety, and the CD3ζ T-cell activation domain. (B) CAR-BCMA expression on the surface of the infusion T cells of patient 10 was detected by staining with a PE-BCMA-Fc protein reagent. The plot is gated on live CD3+ lymphocytes. (C) Demonstrating specificity, the PE-BCMA-Fc reagent did not stain PBMCs that were not transduced with the CAR-BCMA gene. (D) CAR-BCMA T cells of patient 10 were cultured with target cells that either expressed BCMA (BCMA-K562 and RPMI8226) or did not express BCMA (NGFR-K562 and CCRF-CEM). The CAR-BCMA T cells of patient 10 specifically released IFNγ in response to BCMA-expressing target cells in vitro. (E) Patient 8 who had MM that was progressing despite 8 prior lines of therapy obtained a very good partial remission (VGPR) after infusion of CAR-BCMA T cells. Positron emission tomography–computed tomography scans from before and after treatment show elimination of a large number of MM bone lesions. (F) Patient 8’s free κ light chains level decreased after CAR T-cell infusion as the MM entered a VGPR and then increased with progression of the MM. The serum BCMA protein level followed a pattern similar to that of the serum free κ light chains.
Results
CAR-BCMA design and CAR T-cell production
The anti-BCMA CAR used in this work (CAR-BCMA) incorporated the 11D-5-3 anti-BCMA single-chain variable fragment (scFv), a CD28 costimulatory domain, and the CD3−ζ T-cell activation domain (Figure 1A).12 The CAR sequence was expressed by a γ-retroviral vector backbone used in previous work.11,14 CAR-BCMA was consistently expressed on the surface of transduced CD4 and CD8 T cells, and the transduced T cells proliferated extensively in culture (Figure 1B-C; supplemental Tables 1 and 2). A median of 67.0% (range, 45.3-90.8%) of infusion T cells expressed CAR-BCMA. CAR-BCMA T cells specifically recognized BCMA in vitro (Figure 1D).
Patient characteristics and treatment plan
Twelve patients with MM producing a variety of monoclonal proteins were treated. The patients had received a median of 7 prior lines of therapy (Table 1). Prior treatments and cytogenetics of each patient are in supplemental Table 3. No treatment was cancelled because of failure to produce CAR-BCMA T cells, and dose escalation proceeded to the fourth dose level as planned. No antimyeloma therapies were administered after CAR T-cell infusions. Antimyeloma responses for all patients are in Table 1. In screening patients for this study, we stained bone marrow sections from 85 patients by IHC and classified them as follows: 52 BCMA positive and 33 BCMA negative.
Patient information
| Patient . | Monoclonal protein . | Number of prior lines of therapy* . | CAR-BCMA T cells infused (per kg) . | Myeloma response (duration in weeks; + means ongoing)† . |
|---|---|---|---|---|
| 1 | κ light chain only | 7 | 0.3 × 106 | PR (2) |
| 2 | IgA-λ | 10 | 0.3 × 106 | SD (6) |
| 3 | κ light chain only | 8 | 0.3 × 106 | SD (6) |
| 4 | λ light chain only | 7 | 1 × 106 | SD (12) |
| 5 | IgG-κ | 13 | 1 × 106 | SD (4) |
| 6 | IgG-λ | 11 | 1 × 106 | SD (2) |
| 7 | IgG-λ | 6 | 3 × 106 | SD (7) |
| 8 | κ light chain only | 8 | 3 × 106 | VGPR (8) |
| 9 | κ light chain only | 4 | 3 × 106 | SD (16) |
| 10 | IgA-κ | 3 | 9 × 106 | Stringent CR (17) |
| 11 | IgG-λ | 5 | 9 × 106 | VGPR (26+) |
| 12 | IgA-λ | 5 | 3 × 106 | SD (2) |
| Patient . | Monoclonal protein . | Number of prior lines of therapy* . | CAR-BCMA T cells infused (per kg) . | Myeloma response (duration in weeks; + means ongoing)† . |
|---|---|---|---|---|
| 1 | κ light chain only | 7 | 0.3 × 106 | PR (2) |
| 2 | IgA-λ | 10 | 0.3 × 106 | SD (6) |
| 3 | κ light chain only | 8 | 0.3 × 106 | SD (6) |
| 4 | λ light chain only | 7 | 1 × 106 | SD (12) |
| 5 | IgG-κ | 13 | 1 × 106 | SD (4) |
| 6 | IgG-λ | 11 | 1 × 106 | SD (2) |
| 7 | IgG-λ | 6 | 3 × 106 | SD (7) |
| 8 | κ light chain only | 8 | 3 × 106 | VGPR (8) |
| 9 | κ light chain only | 4 | 3 × 106 | SD (16) |
| 10 | IgA-κ | 3 | 9 × 106 | Stringent CR (17) |
| 11 | IgG-λ | 5 | 9 × 106 | VGPR (26+) |
| 12 | IgA-λ | 5 | 3 × 106 | SD (2) |
The number of prior lines of therapy was calculated by adding all discreet lines of therapy received by each patient. An autologous stem cell transplant, including mobilization chemotherapy, conditioning regimen, and any maintenance therapy, was counted as 1 line of therapy. Radiation therapy was counted as a line of therapy. Myeloma staging was conducted according to the International Uniform Response Criteria for Multiple Myeloma.
Response duration was from the time of first documentation of any response to the time of progression. For all patients, response was first documented 2 weeks after CAR-BCMA T-cell infusion.
Toxicities
All patients experienced cytopenias attributable to the conditioning chemotherapy that patients received on this protocol and the extensive prior therapy patients had received before protocol enrollment; Patients 10 and 11 had cytopenias clearly more prolonged than expected from the chemotherapy that they received. Toxicities are listed in Table 2. Toxicities attributable to CAR-BCMA T cells were minimal in patients treated on the 0.3 × 106 CAR+ T cells/kg dose level. On the 1 × 106 CAR+ T cells/kg dose level, patients 4 and 5 had signs and symptoms of CRS including mild fever and tachycardia. Patient 8 had significant fever, tachycardia, and hypotension among other toxicities, but the 3 other patients receiving 3 × 106 CAR+ T cells/kg had mild toxicity. On the 9 × 106 CAR+ T cells/kg dose level, patients 9 and 10 both had a variety of grade 3 and 4 toxicities.
Adverse events
| Patient . | Grade 4* . | Grade 3 . | Grade 2 . |
|---|---|---|---|
| 1 | White blood cell decreased | Hypophosphatemia | Nausea |
| Neutrophil count decreased | Anemia | Headache | |
| Hypocalcemia | |||
| 2 | White blood cell decreased | Anemia | Nausea |
| Neutrophil count decreased | Upper respiratory infection | ||
| Platelet count decreased | |||
| 3 | White blood cell decreased | Anemia | |
| Neutrophil count decreased | |||
| Platelet count decreased | |||
| 4 | White blood cell decreased | Nausea | |
| Hypophosphatemia | Hypocalcemia | ||
| Neutrophil count decreased | Fever | ||
| Atrial fibrillation | |||
| Anemia | |||
| Thromboembolic event | |||
| 5 | White blood cell decreased | aPTT increased | Fever |
| Neutrophil count decreased | Platelet count decreased | ||
| 6 | White blood cell decreased | Hyponatremia | Nausea, vomiting |
| Neutrophil count decreased | Anemia | ||
| 7 | White blood cell decreased | Anemia | |
| Neutrophil count decreased | |||
| 8 | White blood cell decreased | Anemia | Rash, maculopapular |
| Neutrophil count decreased | Fever | Sinus tachycardia | |
| Platelet count decreased | Hypophosphatemia | Dyspnea | |
| Febrile neutropenia | ALT increased | ||
| Hypotension | Hypoalbuminemia, hypocalcemia | ||
| 9 | White blood cell decreased | Hypophosphatemia | Anorexia |
| Neutrophil count decreased | Nausea, vomiting | ||
| Creatine phosphokinase increased† | Anemia | ||
| Diarrhea Upper respiratory infection | |||
| 10 | White blood cell decreased | Fever | Hypomagnesemia |
| Neutrophil count decreased | Anemia | aPTT | |
| Platelet count decreased | Sinus tachycardia | Delirium | |
| Creatine phosphokinase increased | Dyspnea | Hypoalbuminemia | |
| Hypotension | Febrile neutropenia | ALT increased | |
| Catheter-related infection | Urinary tract infection | Hypocalcemia | |
| Sepsis | Hypophosphatemia | Creatinine increased | |
| Hypoxia | Blood bilirubin increased | ||
| Acute kidney injury | Abdominal distention | ||
| AST increased | Thromboembolic event | ||
| Alkalosis | |||
| Hyperuricemia | |||
| Muscle weakness | |||
| Hypokalemia | |||
| 11 | White blood cell decreased | Anemia | Hypoalbuminemia |
| Neutrophil count decreased | Fever | Epistaxis | |
| Platelet count decreased | Delirium | Acute kidney injury | |
| Sinus tachycardia | Creatinine increased | ||
| Hypotension | Hypocalcemia | ||
| Blood bilirubin increased | |||
| Hypoxia | |||
| Hypermagnesemia | |||
| Dyspnea | |||
| Hypernatremia | |||
| Cardiac troponin increased | |||
| Fibrinogen decreased | |||
| Anemia | |||
| Hypophosphatemia | |||
| Creatine phosphokinase increased | |||
| Hypertension AST increased | |||
| 12 | White blood cell decreased | Platelet count decreased | Fever |
| Neutrophil count decreased | Anemia |
| Patient . | Grade 4* . | Grade 3 . | Grade 2 . |
|---|---|---|---|
| 1 | White blood cell decreased | Hypophosphatemia | Nausea |
| Neutrophil count decreased | Anemia | Headache | |
| Hypocalcemia | |||
| 2 | White blood cell decreased | Anemia | Nausea |
| Neutrophil count decreased | Upper respiratory infection | ||
| Platelet count decreased | |||
| 3 | White blood cell decreased | Anemia | |
| Neutrophil count decreased | |||
| Platelet count decreased | |||
| 4 | White blood cell decreased | Nausea | |
| Hypophosphatemia | Hypocalcemia | ||
| Neutrophil count decreased | Fever | ||
| Atrial fibrillation | |||
| Anemia | |||
| Thromboembolic event | |||
| 5 | White blood cell decreased | aPTT increased | Fever |
| Neutrophil count decreased | Platelet count decreased | ||
| 6 | White blood cell decreased | Hyponatremia | Nausea, vomiting |
| Neutrophil count decreased | Anemia | ||
| 7 | White blood cell decreased | Anemia | |
| Neutrophil count decreased | |||
| 8 | White blood cell decreased | Anemia | Rash, maculopapular |
| Neutrophil count decreased | Fever | Sinus tachycardia | |
| Platelet count decreased | Hypophosphatemia | Dyspnea | |
| Febrile neutropenia | ALT increased | ||
| Hypotension | Hypoalbuminemia, hypocalcemia | ||
| 9 | White blood cell decreased | Hypophosphatemia | Anorexia |
| Neutrophil count decreased | Nausea, vomiting | ||
| Creatine phosphokinase increased† | Anemia | ||
| Diarrhea Upper respiratory infection | |||
| 10 | White blood cell decreased | Fever | Hypomagnesemia |
| Neutrophil count decreased | Anemia | aPTT | |
| Platelet count decreased | Sinus tachycardia | Delirium | |
| Creatine phosphokinase increased | Dyspnea | Hypoalbuminemia | |
| Hypotension | Febrile neutropenia | ALT increased | |
| Catheter-related infection | Urinary tract infection | Hypocalcemia | |
| Sepsis | Hypophosphatemia | Creatinine increased | |
| Hypoxia | Blood bilirubin increased | ||
| Acute kidney injury | Abdominal distention | ||
| AST increased | Thromboembolic event | ||
| Alkalosis | |||
| Hyperuricemia | |||
| Muscle weakness | |||
| Hypokalemia | |||
| 11 | White blood cell decreased | Anemia | Hypoalbuminemia |
| Neutrophil count decreased | Fever | Epistaxis | |
| Platelet count decreased | Delirium | Acute kidney injury | |
| Sinus tachycardia | Creatinine increased | ||
| Hypotension | Hypocalcemia | ||
| Blood bilirubin increased | |||
| Hypoxia | |||
| Hypermagnesemia | |||
| Dyspnea | |||
| Hypernatremia | |||
| Cardiac troponin increased | |||
| Fibrinogen decreased | |||
| Anemia | |||
| Hypophosphatemia | |||
| Creatine phosphokinase increased | |||
| Hypertension AST increased | |||
| 12 | White blood cell decreased | Platelet count decreased | Fever |
| Neutrophil count decreased | Anemia |
Adverse event terms from the Common Toxicity Criteria for Adverse Events version 4.02 were used for adverse event grading for this trial, and these terms are used in this table; all greater than grade 1 adverse events are listed regardless of attribution. ALT, alanine amino transferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase.
All patients had grade 4 lymphocyte count decreased, which was expected after the chemotherapy conditioning regimen that the patients received. Lymphocyte count decreased was not listed repetitively for each patient.
Creatine phosphokinase elevation in this patient was almost certainly caused by exercise.
Clinical course of patient 8
Patient 8 received 8 prior lines of therapy including 2 autologous hematopoietic stem cell transplants (ASCTs) prior to enrollment on the CAR-BCMA trial. At the time of protocol enrollment, her MM was producing only κ light chains. She received 3 × 106 CAR-BCMA T cells/kg. One day after the CAR-BCMA T-cell infusion, the patient became febrile. She subsequently developed tachycardia and hypotension; these toxicities resolved by 6 days after CAR T-cell infusion. Two weeks after CAR-BCMA infusion, a positron emission tomography–computed tomography scan showed elimination of extensive MM bone disease that was present before treatment (Figure 1E); in addition, serum free κ light chains became undetectable 2 weeks after treatment (Figure 1F). In agreement with a previous study,37 serum BCMA was detected in patient 8. The serum BCMA level decreased with remission and then increased with progression (Figure 1F). Bone marrow MM cells, which were present before treatment, were not detected by multicolor flow cytometry 2 weeks after CAR-BCMA T-cell infusion. The response to treatment was a very good partial remission (VGPR). This response lasted for 8 weeks. At the time of relapse, 0.3% of bone marrow cells contained the CAR-BCMA gene as measured by qPCR. After progression, patient 8 received 3 different treatment regimens with, at best, transient responses. She died 10 months after her CAR T-cell infusion.
Clinical course of patient 10
Patient 10 was diagnosed with immunoglobulin A (IgA)-κ MM and underwent initial therapy with 6 cycles of cyclophosphamide, bortezomib, and dexamethasone (CyBorD), which resulted in a partial remission (PR). The patient received high-dose melphalan (200 mg/m2) followed by an ASCT. Three months after the ASCT, he had progressive MM, and 90% of his bone marrow cells were plasma cells. This rapid progression of MM after ASCT demonstrated that the MM was highly resistant to chemotherapy. Patient 10 then received 2 cycles of carfilzomib, lenalidomide, and dexamethasone, which resulted in a partial remission (PR) lasting <2 months. Next, patient 10 enrolled in the CAR-BCMA trial; he had rapidly progressive MM. More than 90% of the cells in the patient’s hypercellular bone marrow were plasma cells, and the plasma cells expressed uniform but dim BCMA (Figure 2A,B,D).
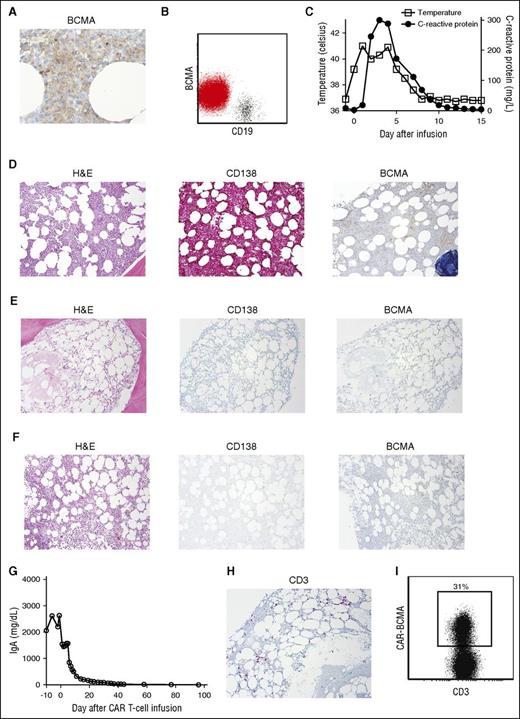
Patient 10 entered a stringent complete remission. (A) Before treatment, patient 10’s bone marrow was stained for BCMA by IHC. BCMA expression was uniform but dim on the MM cells (500×). (B) Flow cytometry of patient 10’s bone marrow showed dim but uniform BCMA expression on the CD19-negative malignant plasma cells (red), whereas BCMA expression was absent on CD19+ B lymphocytes (black). The plot shows a combination of the plasma cell gate (CD38bright, CD138+, CD45dim-negative) and the mature B-cell gate (CD45+, CD19+ lymphocytes). (C) Four hours after CAR-BCMA infusion, patient 10 became febrile. He was febrile for 7 days. The plot shows the maximum temperature for each day. The CRP was elevated. (D) Before initiation of protocol treatment, patient 10 had a hypercellular bone marrow (hematoxylin and eosin). Bone marrow cells were ≥90% plasma cells as shown by CD138 staining. BCMA expression was dim but present. (E) Four weeks after CAR T-cell infusion, the bone marrow was hypocellular, and bone marrow plasma cells were completely absent as shown by the negative CD138 and BCMA staining. (F) Eight weeks after CAR T-cell infusion, the bone marrow was recovering with an overall cellularity of 25%, and plasma cells remained absent. Magnification for D-F was 40×. (G) Immediately before CAR-BCMA infusion, the patient had a serum IgA level of 2633 mg/dL. After CAR T-cell infusion on day 0, serum IgA decreased until reaching undetectable levels. (H) IHC staining of patient 10’s bone marrow showed an infiltrate of CD3+ T cells 4 weeks after CAR T-cell infusion. (I) Flow cytometry with a PE-BCMA-Fc reagent showed that 31% of bone marrow T cells were CAR-BCMA T cells 4 weeks after CAR T-cell infusion. The plot is gated on CD45+ and CD3+ lymphocytes.
Patient 10 entered a stringent complete remission. (A) Before treatment, patient 10’s bone marrow was stained for BCMA by IHC. BCMA expression was uniform but dim on the MM cells (500×). (B) Flow cytometry of patient 10’s bone marrow showed dim but uniform BCMA expression on the CD19-negative malignant plasma cells (red), whereas BCMA expression was absent on CD19+ B lymphocytes (black). The plot shows a combination of the plasma cell gate (CD38bright, CD138+, CD45dim-negative) and the mature B-cell gate (CD45+, CD19+ lymphocytes). (C) Four hours after CAR-BCMA infusion, patient 10 became febrile. He was febrile for 7 days. The plot shows the maximum temperature for each day. The CRP was elevated. (D) Before initiation of protocol treatment, patient 10 had a hypercellular bone marrow (hematoxylin and eosin). Bone marrow cells were ≥90% plasma cells as shown by CD138 staining. BCMA expression was dim but present. (E) Four weeks after CAR T-cell infusion, the bone marrow was hypocellular, and bone marrow plasma cells were completely absent as shown by the negative CD138 and BCMA staining. (F) Eight weeks after CAR T-cell infusion, the bone marrow was recovering with an overall cellularity of 25%, and plasma cells remained absent. Magnification for D-F was 40×. (G) Immediately before CAR-BCMA infusion, the patient had a serum IgA level of 2633 mg/dL. After CAR T-cell infusion on day 0, serum IgA decreased until reaching undetectable levels. (H) IHC staining of patient 10’s bone marrow showed an infiltrate of CD3+ T cells 4 weeks after CAR T-cell infusion. (I) Flow cytometry with a PE-BCMA-Fc reagent showed that 31% of bone marrow T cells were CAR-BCMA T cells 4 weeks after CAR T-cell infusion. The plot is gated on CD45+ and CD3+ lymphocytes.
Patient 10 received an infusion of 9 × 106 CAR-BCMA T cells/kg. Four hours after the CAR-BCMA T-cell infusion, he became febrile and tachycardic. The patient was febrile for 7 days with a maximum temperature of 41.0°C (Figure 2C). The C-reactive protein (CRP) was elevated. The patient had hypotension requiring treatment with norepinephrine. The IL-6 receptor antagonist tocilizumab was administered on day 3 and again on day 4 after CAR T-cell infusion. The patient had an increase in serum creatine phosphokinase to >20 000 U/L (normal, 39-308 U/L) that was associated with muscle weakness. Within 15 days after CAR-BCMA T-cell infusion, all toxicities except for cytopenias and muscle weakness had resolved to grade 1 or less. The muscle weakness resolved to baseline over 2 months.
Patient 10 experienced pancytopenia associated with a hypocellular bone marrow (Figure 2E). He had an absolute neutrophil count (ANC) of 370 immediately before the CAR-BCMA T-cell infusion. The ANC remained <500 for 40 days after CAR-BCMA T-cell infusion, and the patient had an episode of bacteremia associated with transient hypotension during this neutropenic period. The patient also experienced transfusion-dependent thrombocytopenia for 9 weeks after CAR-BCMA infusion. Fourteen weeks after CAR T-cell infusion, patient 10 had a normal ANC, and he had not required a platelet transfusion in >6 weeks, although his platelet count remained decreased at 43 000/μL.
During the first week after CAR-BCMA infusion, we noted a striking decrease in patient 10’s serum IgA level (Figure 2G). Serum IgA became undetectable and remained undetectable 14 weeks after CAR-BCMA T-cell infusion. A dramatic eradication of MM from the bone marrow occurred. IHC for the plasma cell marker CD138 showed a complete absence of bone marrow plasma cells 4 and 8 weeks after CAR-BCMA T-cell infusion (Figure 2E-F). Four weeks after CAR T-cell infusion, a population of T cells was detected in the bone marrow (Figure 2H); 31% of bone marrow T cells expressed CAR-BCMA by flow cytometry (Figure 2I). The serum and urine immunofixation electrophoresis tests for monoclonal IgA-κ became negative after treatment and remained negative 14 weeks after CAR-BCMA T cell infusion. Fourteen weeks after CAR-BCMA T-cell infusion, multicolor flow cytometry did not detect MM cells in the bone marrow, which confirmed a stringent complete remission (CR). As expected, normal plasma cells were also absent from the bone marrow 14 weeks after CAR-BCMA infusion. Nineteen weeks after infusion, the serum immunofixation electrophoresis remained negative, but a monoclonal IgA-κ protein was detected in the urine. This indicated a relapse of MM. Subsequently, BCMA+ malignant plasma cells were detected in the bone marrow; CAR-BCMA T cells were not detected in the bone marrow at the time of relapse.
Clinical course of patient 11
Patient 11 received 5 prior lines of therapy before enrolling on the CAR-BCMA protocol. His most recent therapy prior to enrollment was CyBorD; during his only cycle of this treatment, patient 11’s MM progressed. Before CAR-BCMA infusion, 80% of the cells in the patient’s hypercellular bone marrow were BCMA+ plasma cells (Figure 3A,B,D). Four hours after infusion of 9 × 106 CAR-BCMA T cells/kg, he developed a sustained fever that was associated with an elevated CRP (Figure 3C). Worsening tachycardia, hypoxemia, and dyspnea developed over the next day, so 25 hours after the CAR T-cell infusion, tocilizumab was administered. This patient also had grade 3 delirium without other neurologic toxicities. Four hours after the tocilizumab was administered, the dyspnea improved, and the temperature and heart rate decreased temporarily. A second dose of tocilizumab was administered 5 days after the CAR T-cell infusion. Patient 11 had adrenal insufficiency at the time of protocol enrollment, and he received stress-dose intravenous hydrocortisone at doses varying from 25 to 75 mg every 8 to 12 hours between day 0 and day 13 after CAR T-cell infusion.
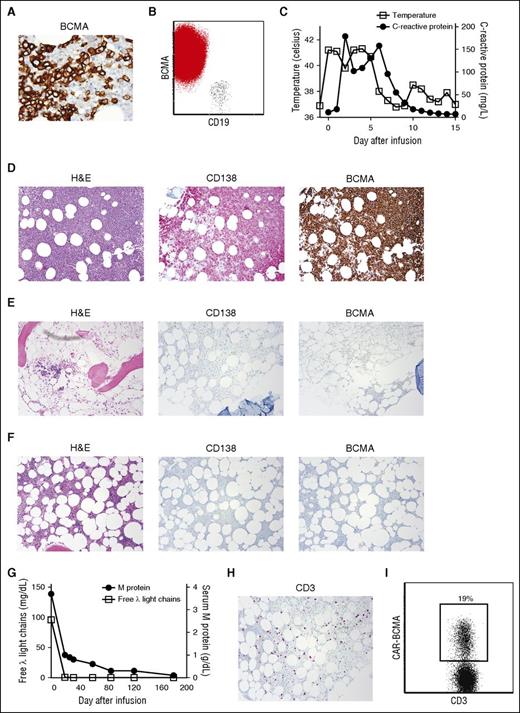
MM was eliminated from the bone marrow of patient 11 after CAR-BCMA infusion. (A) Before treatment, BCMA expression was uniform and strong on the MM cells of patient 11 (500×). (B) Flow cytometry showed strong uniform BCMA expression on CD19-negative malignant plasma cells (red), whereas BCMA expression was absent on CD19+ B lymphocytes (black). The plot shows a combination of the plasma cell gate (CD38bright, CD138+, CD45dim-negative) and the mature B-cell gate (CD45+, CD19+ lymphocytes). (C) Four hours after CAR-BCMA infusion, patient 11 became febrile. He was febrile until 5 days after the CAR T-cell infusion. The plot shows the maximum temperature for each day. The CRP was also elevated. (D) Before protocol treatment, patient 11 had a hypercellular bone marrow (hematoxylin and eosin ). Bone marrow cells were 80% plasma cells as shown by CD138 staining. (E) Four weeks after CAR T-cell infusion, the bone marrow was hypocellular, and bone marrow plasma cells were completely absent as shown by negative CD138 and BCMA staining. (F) Eight weeks after CAR T-cell infusion, the marrow was recovering with an overall cellularity of 20%; plasma cells remained absent. Magnification for D-F was 40×. (G) Before protocol treatment, the patient’s serum λ free light chains level was 95.9 mg/dL. The λ free light chains decreased to an undetectable level after CAR-BCMA T-cell infusion. Before treatment, the patient had an IgG λ M-protein level of 3.7 g/dL. The M-protein decreased after treatment. (H) Patient 11’s bone marrow contained an infiltrate of CD3+ T cells 4 weeks after CAR T-cell infusion. (I) Flow cytometry with PE-BCMA-Fc showed that 19% of bone marrow T cells were CAR-BCMA T cells 4 weeks after CAR T-cell infusion. The plot is gated on CD45+, CD3+ lymphocytes.
MM was eliminated from the bone marrow of patient 11 after CAR-BCMA infusion. (A) Before treatment, BCMA expression was uniform and strong on the MM cells of patient 11 (500×). (B) Flow cytometry showed strong uniform BCMA expression on CD19-negative malignant plasma cells (red), whereas BCMA expression was absent on CD19+ B lymphocytes (black). The plot shows a combination of the plasma cell gate (CD38bright, CD138+, CD45dim-negative) and the mature B-cell gate (CD45+, CD19+ lymphocytes). (C) Four hours after CAR-BCMA infusion, patient 11 became febrile. He was febrile until 5 days after the CAR T-cell infusion. The plot shows the maximum temperature for each day. The CRP was also elevated. (D) Before protocol treatment, patient 11 had a hypercellular bone marrow (hematoxylin and eosin ). Bone marrow cells were 80% plasma cells as shown by CD138 staining. (E) Four weeks after CAR T-cell infusion, the bone marrow was hypocellular, and bone marrow plasma cells were completely absent as shown by negative CD138 and BCMA staining. (F) Eight weeks after CAR T-cell infusion, the marrow was recovering with an overall cellularity of 20%; plasma cells remained absent. Magnification for D-F was 40×. (G) Before protocol treatment, the patient’s serum λ free light chains level was 95.9 mg/dL. The λ free light chains decreased to an undetectable level after CAR-BCMA T-cell infusion. Before treatment, the patient had an IgG λ M-protein level of 3.7 g/dL. The M-protein decreased after treatment. (H) Patient 11’s bone marrow contained an infiltrate of CD3+ T cells 4 weeks after CAR T-cell infusion. (I) Flow cytometry with PE-BCMA-Fc showed that 19% of bone marrow T cells were CAR-BCMA T cells 4 weeks after CAR T-cell infusion. The plot is gated on CD45+, CD3+ lymphocytes.
Patient 11’s serum IgG-λ M-protein started to decrease after CAR-BCMA T-cell infusion and decreased to the point that it was not quantifiable at last follow-up 28 weeks after CAR T-cell infusion. The only evidence of MM at last follow-up was the serum IgG-λ M-protein that was detectable only by immunofixation electrophoresis. The serum free λ light chains that were another marker of the patient’s MM became undetectable early after treatment (Figure 3G). Patient 11’s most recent formal myeloma response was VGPR. A bone marrow biopsy 4 weeks after the CAR T-cell infusion revealed a hypocellular bone marrow and complete elimination of bone marrow plasma cells (Figure 3E). The patient was platelet transfusion dependent from day 2 until day 27 after CAR T-cell infusion. Eight weeks after the CAR T-cell infusion, bone marrow cellularity was recovering, and plasma cells remained completely absent (Figure 3F). Absence of MM cells and normal plasma cells from the bone marrow was confirmed by multicolor flow cytometry 4, 8, and 25 weeks after CAR T-cell infusion. Four weeks after CAR T-cell infusion, a large population of T cells was detected in the bone marrow (Figure 3H); 19% of bone marrow T cells expressed CAR-BCMA by flow cytometry (Figure 3I). Less than 8 weeks after the CAR T-cell infusion, all of patient 11’s toxicities except decreased lymphocyte count had improved to grade 1 or resolved, and he had returned to full-time employment. In accordance with continued absence of plasma cells in patient 11’s bone marrow, the serum immunoglobulin levels steadily decreased after CAR-BCMA infusion. Twenty-eight weeks after CAR T-cell infusion, the serum IgG was 388 mg/dL (normal range, 700-1600 mg/dL), the serum IgA was below detectable limits, and the serum IgM was 15 mg/dL (normal range, 40-230 mg/dL). Because of the low immunoglobulin levels, we initiated immunoglobulin replacement with intravenous IgG.
Quantifying blood CAR-BCMA T cells
We measured the number of CAR-BCMA+ cells in the blood of all patients with a qPCR assay that was able to specifically detect CAR-BCMA+ PBMCs (Figure 4A). Although CAR-BCMA+ cells could be detected in the blood of all patients, the patients on the lower 2 dose levels had low levels of blood CAR-BCMA+ cells, and the patients with the most impressive antimyeloma responses, patients 8, 10, and 11, had the highest peak CAR-BCMA+ cell levels. By 3 months after infusion, blood CAR-BCMA+ cell levels had decreased to 0.1% of PBMCs or less in all patients.
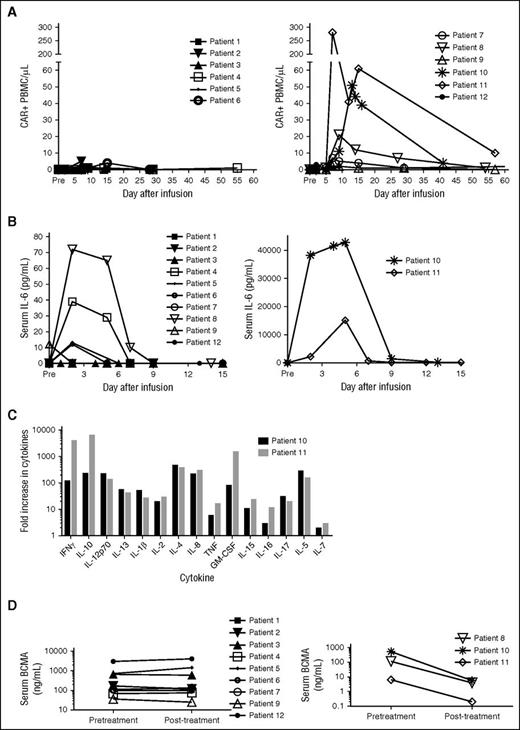
Patients with the most impressive antimyeloma responses had the highest levels of blood CAR-BCMA+ cells. (A) CAR-BCMA+ PBMCs were quantified in the blood of patients by qPCR. Patients treated on the 2 lower dose levels had low levels of CAR-BCMA+ cells in their blood (left). Patients treated on the higher 2 dose levels had higher levels of CAR-BCMA+ cells in their blood (right). Patients 8, 10, and 11 had the best antimyeloma responses and the highest levels of blood CAR-BCMA+ cells. The results are presented as the absolute number of CAR+ PBMC/μL of blood. Pre stands for pretreatment. (B) IL-6 was measured in the serum of all patients. Patients 10 and 11 (right) had the most severe cytokine-release syndrome, and these patients also had the highest serum IL-6 levels. Serum IL-6 levels of the other patients are shown on the left. Note the different y-axis scales on the left and right graphs. (C) Five days after CAR-BCMA T cell infusion, serum levels of 15 cytokines were measured in the blood of patient 10 and patient 11. Results are expressed as fold increase over pretreatment levels. (D) Serum soluble BCMA of all patients was measured by ELISA pretreatment and posttreatment. The posttreatment serum samples were obtained 22 to 29 days after CAR T-cell infusion for all patients except patient 12. The posttreatment sample of patient 12 was obtained 13 days after CAR T-cell infusion. All patients had detectable serum BCMA. In the patients obtaining the most impressive antimyeloma responses, Patients 8, 10, and 11, serum BCMA decreased posttreatment (right); in contrast, serum BCMA did not decrease substantially posttreatment in the other patients (left).
Patients with the most impressive antimyeloma responses had the highest levels of blood CAR-BCMA+ cells. (A) CAR-BCMA+ PBMCs were quantified in the blood of patients by qPCR. Patients treated on the 2 lower dose levels had low levels of CAR-BCMA+ cells in their blood (left). Patients treated on the higher 2 dose levels had higher levels of CAR-BCMA+ cells in their blood (right). Patients 8, 10, and 11 had the best antimyeloma responses and the highest levels of blood CAR-BCMA+ cells. The results are presented as the absolute number of CAR+ PBMC/μL of blood. Pre stands for pretreatment. (B) IL-6 was measured in the serum of all patients. Patients 10 and 11 (right) had the most severe cytokine-release syndrome, and these patients also had the highest serum IL-6 levels. Serum IL-6 levels of the other patients are shown on the left. Note the different y-axis scales on the left and right graphs. (C) Five days after CAR-BCMA T cell infusion, serum levels of 15 cytokines were measured in the blood of patient 10 and patient 11. Results are expressed as fold increase over pretreatment levels. (D) Serum soluble BCMA of all patients was measured by ELISA pretreatment and posttreatment. The posttreatment serum samples were obtained 22 to 29 days after CAR T-cell infusion for all patients except patient 12. The posttreatment sample of patient 12 was obtained 13 days after CAR T-cell infusion. All patients had detectable serum BCMA. In the patients obtaining the most impressive antimyeloma responses, Patients 8, 10, and 11, serum BCMA decreased posttreatment (right); in contrast, serum BCMA did not decrease substantially posttreatment in the other patients (left).
Serum IL-6 and other cytokines were elevated in patients with signs of toxicity attributable to CAR T-cells
Serum IL-6 was measured pretreatment and at several time points after treatment (Figure 4B). The highest levels of serum IL-6 were detected during the first 5 days after CAR-BCMA T-cell infusion, which was also the time when patients were experiencing the most toxicity. Patients 10 and 11 had by far the highest levels of serum IL-6 and the most severe toxicity. In addition to IL-6, many other cytokines were highly elevated in the serum of patients 10 and 11 (Figure 4C); serum IFNγ peaked at the same time as IL-6.
Soluble serum BCMA
We detected soluble BCMA in the serum of all 12 patients before treatment (Figure 4D). Serum BCMA decreased after CAR-BCMA T-cell infusion in the patients with the most impressive antimyeloma responses, patients 8, 10, and 11; in contrast, serum BCMA did not decrease substantially after CAR-BCMA T-cell infusion in the other patients.
BCMA expression at progression
Progression of BCMA-negative MM is a potential problem after CAR-BCMA T-cell therapies. We assessed bone marrow MM cells for BCMA expression after CAR-BCMA T-cell infusion by IHC; the before and after treatment BCMA expressions are summarized in supplemental Table 4. A partial loss of BCMA expression by malignant plasma cells was detected only in patient 7 (supplemental Figure 1).
CAR-BCMA T-cell phenotype
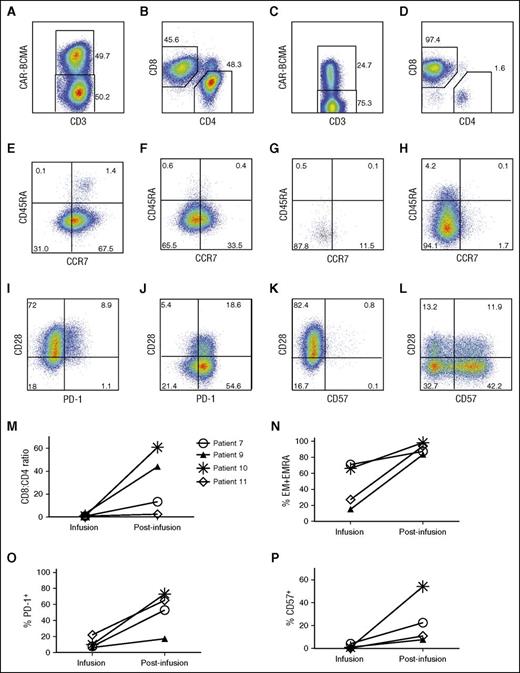
For all 12 patients, the median ratio of CD8+ to CD4+ CAR+ T cells in the infusion cells was 1.1 (range, 0.3-10.9; supplemental Table 4). Sufficient CAR-BCMA T cells were available for a phenotypic analysis of blood T cells from 4 patients after infusion (Figure 5). Among these 4 patients, the median ratio of CD8+ to CD4+ CAR+ T cells in the infusion cells was 0.9. In contrast, the persisting CAR+ T cells in the blood of the same patients after infusion were mainly CD8+ T cells with a median CD8+ to CD4+ ratio of 28.8 (range, 2.5-60.9; Figure 5M). The expression of C-C chemokine receptor type 7 (CCR7) and CD45RA is used to divide T cells into 4 categories: naïve, central memory (CM), effector memory (EM), and effector memory RA (EMRA). Compared with naïve and CM T cells, EM and EMRA T cells are more differentiated with less proliferative potential.38 CCR7 and CD45RA expression data of the infusion CAR-BCMA T cells of all patients are in supplemental Tables 5 and 6. Programmed cell death protein-1 (PD-1) is an inhibitory T-cell receptor important in T-cell exhaustion.39 CD57 is a marker of highly differentiated T cells with reduced proliferative capacity.40,41 In the 4 patients with sufficient CAR+ T cells for phenotypic analysis of blood T cells after infusion, CAR T cells acquired a more differentiated phenotype with an increase in EM and EMRA T cells after infusion (Figure 5N). Increases in expression of PD-1 and CD57 provided further evidence that CAR-BCMA T cells acquired a highly differentiated phenotype after infusion (Figure 5O-P).
CAR-BCMA T cells became more differentiated after infusion. For A-L, cells from patient 10 are shown. All plots are gated on live lymphocytes, and the numbers on the plots are the percentages of cells in the indicated regions of the plots. Staining for CAR-BCMA was performed with PE-BCMA-Fc. All postinfusion samples were PBMCs that were collected 16 days after CAR-BCMA T-cell infusion. (A) Cells from the time of infusion (infusion cells) were stained for CD3 and CAR-BCMA. (B) This plot is gated on the CD3+CAR+ infusion cells. (C) Postinfusion cells were stained for CD3 and CAR-BCMA. (D) This plot is gated on postinfusion CD3+CAR+ cells. CAR T cells after infusion were predominantly CD8+. (E) CCR7 and CD45RA expression of CD3+CD4+CAR+ infusion cells is shown. (F) CCR7 and CD45RA expression of CD3+CD8+CAR+ infusion cells is shown. (G) CCR7 and CD45RA expression of postinfusion blood CD3+CD4+CAR+ T cells. (H) CCR7 and CD45RA expression of postinfusion blood CD3+CD8+CAR+ T cells. (I) CD3+CD8+CAR+ infusion cell PD-1 and CD28 expression are shown. (J) PD-1 and CD28 expression is shown on postinfusion blood CD3+CD8+CAR+ T cells. (K) CD57 and CD28 expression on CD3+CD8+CAR+ infusion cells is shown. (L) CD57 and CD28 expression of postinfusion blood CD3+CD8+CAR+ T cells is shown. For M-P, results are shown for all 4 patients with sufficient blood CAR-BCMA+ T cells from 7 to 16 days after infusion for analysis. Gating and analysis were performed as described above in A-L. The results are all for live CD3+CAR+ cells. The symbols shown in M correspond to the same patients for M-P. (M) The CD8+ to CD4+ ratio of CAR-BCMA T cells increased for all 4 assessable patients between infusion and the time the postinfusion samples were collected. The percentage of CD8+ CAR-BCMA T cells with (N) EM or EMRA phenotypes, (O) PD-1 expression, and (P) CD57 expression increased in all 4 assessable patients between infusion and the time a postinfusion sample was collected.
CAR-BCMA T cells became more differentiated after infusion. For A-L, cells from patient 10 are shown. All plots are gated on live lymphocytes, and the numbers on the plots are the percentages of cells in the indicated regions of the plots. Staining for CAR-BCMA was performed with PE-BCMA-Fc. All postinfusion samples were PBMCs that were collected 16 days after CAR-BCMA T-cell infusion. (A) Cells from the time of infusion (infusion cells) were stained for CD3 and CAR-BCMA. (B) This plot is gated on the CD3+CAR+ infusion cells. (C) Postinfusion cells were stained for CD3 and CAR-BCMA. (D) This plot is gated on postinfusion CD3+CAR+ cells. CAR T cells after infusion were predominantly CD8+. (E) CCR7 and CD45RA expression of CD3+CD4+CAR+ infusion cells is shown. (F) CCR7 and CD45RA expression of CD3+CD8+CAR+ infusion cells is shown. (G) CCR7 and CD45RA expression of postinfusion blood CD3+CD4+CAR+ T cells. (H) CCR7 and CD45RA expression of postinfusion blood CD3+CD8+CAR+ T cells. (I) CD3+CD8+CAR+ infusion cell PD-1 and CD28 expression are shown. (J) PD-1 and CD28 expression is shown on postinfusion blood CD3+CD8+CAR+ T cells. (K) CD57 and CD28 expression on CD3+CD8+CAR+ infusion cells is shown. (L) CD57 and CD28 expression of postinfusion blood CD3+CD8+CAR+ T cells is shown. For M-P, results are shown for all 4 patients with sufficient blood CAR-BCMA+ T cells from 7 to 16 days after infusion for analysis. Gating and analysis were performed as described above in A-L. The results are all for live CD3+CAR+ cells. The symbols shown in M correspond to the same patients for M-P. (M) The CD8+ to CD4+ ratio of CAR-BCMA T cells increased for all 4 assessable patients between infusion and the time the postinfusion samples were collected. The percentage of CD8+ CAR-BCMA T cells with (N) EM or EMRA phenotypes, (O) PD-1 expression, and (P) CD57 expression increased in all 4 assessable patients between infusion and the time a postinfusion sample was collected.
Discussion
We demonstrated that CAR-BCMA T cells can eliminate large burdens of treatment-resistant MM. The prior treatment histories of patients 10 and 11 demonstrated the chemotherapy resistance of their MM. Prior to enrollment on the CAR-BCMA protocol, patient 10 only obtained a PR after 6 cycles of CyBorD therapy, and he had 90% bone marrow plasma cells 3 months after high-dose melphalan and ASCT. High-dose melphalan is a much more effective antimyeloma therapy than the low-dose cyclophosphamide administered immediately prior to CAR-BCMA T-cell infusions. Patient 11 had MM that was refractory to CyBorD therapy. Fludarabine, which was administered prior to CAR-BCMA T-cell infusions, has been shown to have essentially no activity against MM.42 All in all, the antimyeloma responses after CAR-BCMA infusion in patients 10 and 11 cannot be attributed to the chemotherapy administered prior to CAR-BCMA T-cell infusions.
One of the advantages of T-cell therapies for MM is that such therapies are very different than all current therapies. Although CAR T-cell therapies for MM are clearly in early stages of development, it is possible that these therapies will be able to eliminate MM cells that are resistant to current MM therapies. Our results are distinct from those of other recent publications that either described human-leukocyte-antigen–restricted T cells as MM therapy43 or reported infusion of anti-CD19 CAR T cells into a single patient after an ASCT to prevent MM relapse.44
We have not observed unexpected damage to nonhematopoietic organs after infusion of CAR-BCMA T cells. We observed clinical signs of cytokine-mediated toxicity in patients with elevated serum levels of IL-6 and other cytokines (Figure 4B-C).14,24 The toxicities experienced by patients on this trial, including elevated creatine phosphokinase levels,23 were strikingly similar to the toxicities experienced by acute lymphoblastic leukemia (ALL) patients on clinical trials of anti-CD19 CAR T cells.17,23 As observed with patient 10 on this trial, we previously observed elevations in creatine phosphokinase and muscle weakness in patients on a trial of anti-CD19 CAR T cells.23 The creatine phosphokinase elevations only occurred in patient with severe CRS with high body temperatures.
Loss of normal plasma cells after CAR-BCMA T-cell infusions is an expected toxicity because normal plasma cells express BCMA.12 The low polyclonal immunoglobulin levels of Patient 11 show that, at least in some cases, immunoglobulin replacement will be indicated in patients receiving anti-BCMA CAR T cells.
We have previously shown that anti-CD19 CAR T cells administered without chemotherapy can cause cytopenias in patients with high disease-burden ALL.23 Of the patients receiving CAR-BCMA T cells, patient 10 experienced prolonged pancytopenia and patient 11 experienced prolonged thrombocytopenia. We hypothesize that the cytopenias experienced by these 2 patients were a cytokine-mediated phenomenon caused by high levels of cytokines produced when CAR-BCMA T cells contacted high burdens of bone marrow MM; however, bystander perforin/granzyme-mediated killing of hematopoietic cells could also be speculated. Both of these patients were cytopenic prior to CAR-BCMA T-cell infusion, which probably contributed to the severity of the cytopenias after CAR-BCMA T-cell infusion (supplemental Table 7). The chemotherapy that patients received as part of this protocol is expected to cause cytopenias, and the extensive therapy that the patients had received prior to protocol enrollment probably contributed to chronic bone marrow impairment in some patients. We never observed BCMA expression on any bone marrow cells except plasma cells after performing IHC staining on bone marrows from 85 patients.
CAR-BCMA T cells can eliminate MM cells even when high concentrations of soluble BCMA are present in the blood of patients (Figure 4D). In addition to being an important finding for CAR-BCMA T-cell therapy, this finding is important because many other promising CAR target antigens are also present in soluble form in blood and at tumor sites.45,46 Serum BCMA served as a tumor marker because substantial decreases in serum BCMA occurred in the 3 patients with the most impressive antimyeloma responses (Figure 4D).
Although the response rates for anti-BCMA CAR T cells against MM reported here are not as high as previous reports of anti-CD19 CAR T cells against B-cell malignancies,16,17,19,25 the number of patients treated with anti-BCMA CARs is quite small. Compared with the doses of anti-CD19 CAR T cells generally required for efficacy and toxicity in our experience (1-2 × 106/kg),19 higher doses of anti-BCMA CAR T cells (3-9 × 106 CAR+ T cells/kg) were required to elicit substantial antimalignancy responses and toxicity. This difference could be due to many factors including weaker BCMA expression on malignant cells in comparison with CD19, differences in CAR design between anti-CD19 CARs used previously and the anti-BCMA CAR used in this work, and the presence of soluble BCMA in serum and at tumor sites.
We chose to focus on improving anti-BCMA CAR T-cell therapies by simultaneously pursuing several different projects. We believe that optimizing CAR design is currently the most important way to improve CAR T-cell therapies; therefore, we are pursuing different projects aimed at finding the optimal design for anti-BCMA CARs. We are collaborating on a clinical trial of a 4-1BB–containing anti-BCMA CAR. For the trial using the CD28-containing CAR that is the subject of this report, we decided to administer different doses based on disease burden. Because of the toxicities experienced by patients 10 and 11, we will only administer 9 × 106 CAR-BCMA T cells/kg to patients with <50% bone marrow plasma cells; patients with >50% bone marrow plasma cells will receive 3 × 106 CAR-BCMA T cell/kg. We are also undertaking a preclinical project that will examine the hinge and transmembrane regions of anti-BCMA CARs, and we are attempting to develop a fully human anti-BCMA CAR.
These results demonstrate for the first time that CAR T-cells targeting an antigen other than CD19 can induce complete remissions of a hematologic malignancy. Importantly, we have shown that CAR-BCMA T cells have powerful activity against MM that was resistant to standard therapies. These results should encourage further efforts to enhance anti-BCMA CAR T cell therapies. The striking activity of anti-BCMA CAR T cells against MM indicates that CAR T cells targeting BCMA have great potential to be an effective new treatment of MM. Further development of anti-BCMA CAR T-cell therapies is a very promising area of research.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jo Lynn Procter and the rest of the Cell Processing Service, Department of Transfusion Medicine, Clinical Center, National Institutes of Health (NIH), for cell production; the Experimental Transplantation and Immunology Branch, National Cancer Center (NCI) clinical service, the Clinical Center, NIH intensive care unit staff, and the 3 Northeast Clinical Center NIH nursing units for patient care; Steven Rosenberg for assistance with γ-retroviral vector production; Rashmika Patel for clinical regulatory work; Monica Cho for processing blood samples; and Constance Yuan for conducting flow cytometry.
This work was supported by intramural funding of the Center for Cancer Research, National Cancer Institute, NIH. In addition, the NCI has a Cooperative Research and Development Agreement with bluebird bio, Inc, which supports development of anti-BCMA CAR T-cell therapies. J.N.K. is the NCI Principle Investigator of this Research Agreement.
Authorship
Contribution: all authors read and approved the manuscript; S.A.A., R.E.G., D.F.S., S.A.F., F.T.H., and J.N.K. planned the trial and experiments; S.A.F., J.N.K., V.S., V.S.F., J.J.R., and I.M. conducted experiments; S.A.A., V.S., M.W., I.M., J.N.K., J.N.B., B.G.H., J.J.R., F.T.H., and M.S.-S. analyzed data; S.A.A. and J.N.K. wrote the paper; J.N.K. wrote the first version of the manuscript; and S.A.A., M.W., J.N.B., B.G.H., J.N.K., and R.E.G. provided patient care.
Conflict-of-interest disclosure: J.N.K. has patent applications for anti-BCMA CARs and receives royalties for anti-BCMA CARs licensed by the NCI. The remaining authors declare no competing financial interests.
The current affiliation for S.A.A. is Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD.
Correspondence: James N. Kochenderfer, National Institutes of Health, Building 10, Room 3E-3330, Bethesda, MD 20892; e-mail: kochendj@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal