Key Points
Under current treatment approaches, patients with LA GVHD have poor overall and failure-free survival.
Levels of AREG are elevated in LA GVHD, and the AREG/EGF ratio is predictive of overall survival and nonrelapse mortality in LA GVHD.
Abstract
Late acute (LA) graft-versus-host disease (GVHD) is persistent, recurrent, or new-onset acute GVHD symptoms occurring >100 days after allogeneic hematopoietic cell transplantation (HCT). The aim of this analysis is to describe the onset, course, morbidity, and mortality of and examine angiogenic factors associated with LA GVHD. A prospective cohort of patients (n = 909) was enrolled as part of an observational study within the Chronic GVHD Consortium. Eighty-three patients (11%) developed LA GVHD at a median of 160 (interquartile range, 128-204) days after HCT. Although 51 out of 83 (61%) achieved complete or partial response to initial therapy by 28 days, median failure-free survival was only 7.1 months (95% confidence interval, 3.4-19.1 months), and estimated overall survival (OS) at 2 years was 56%. Given recently described alterations of circulating angiogenic factors in classic acute GVHD, we examined whether alterations in such factors could be identified in LA GVHD. We first tested cases (n = 55) and controls (n = 50) from the Chronic GVHD Consortium and then validated the findings in 37 cases from Mount Sinai Acute GVHD International Consortium. Plasma amphiregulin (AREG; an epidermal growth factor [EGF] receptor ligand) was elevated, and an AREG/EGF ratio at or above the median was associated with inferior OS and increased nonrelapse mortality in both cohorts. Elevation of AREG was detected in classic acute GVHD, but not chronic GVHD. These prospective data characterize the clinical course of LA GVHD and demonstrate alterations in angiogenic factors that make LA GVHD biologically distinct from chronic GVHD.
Introduction
Graft-versus-host disease (GVHD)–related mortality remains a source of failure after allogeneic hematopoietic cell transplantation (HCT), and our current understanding of late acute (LA) GVHD is limited. Although reclassification following the NIH Consensus Conference improved diagnostic criteria,1 subsequent retrospective analyses have not uniformly found that LA GVHD has a prognosis distinct from acute or chronic GVHD.2-7 Improved understanding of LA GVHD is needed prior to development of clinical trials aimed at the prevention and/or treatment of this syndrome.
The pathogenesis of LA GVHD is unknown, as onset is removed from conditioning regimen–induced tissue damage and signals that initiate classic acute GVHD.8 Data support that circulating angiogenic factors are altered at the onset of classic acute GVHD.9 Inflammation-associated follistatin levels are elevated and associated with mortality, whereas wound-healing–associated epidermal growth factor (EGF) levels are low and decrease further in steroid-refractory disease.9 We sought to examine these factors in the setting of LA GVHD. Given the association of low EGF with steroid-refractory acute GVHD, we expanded the study of EGF receptor ligands to include amphiregulin (AREG) and heparin-binding (HB) EGF. We also examined EGF receptor ligand sheddases (disintegrin and metalloproteinase domain-containing 10 [ADAM10] and ADAM17), given the importance of EGF receptor ligand ectodomain shedding in regeneration after injury.10 We also evaluated the prognostic significance of follistatin in the setting of LA GVHD and included activin A to investigate whether follistatin may be elevated in response to activin A–driven inflammation.11
We present the first prospective analysis of LA GVHD, examining its incidence, characteristics, therapeutic response, and outcomes. These data provide important benchmarks for the design of future clinical trials. In addition, we present analysis of circulating angiogenic factors that may aid in diagnosis, risk stratification, and translational therapies in LA GVHD.
Methods
A prospective cohort of 909 patients was enrolled between March 2011 and May 2014 at 13 centers. The protocol was approved by the institutional review board at each site. Patients were enrolled pre-HCT or up to 121 days post-HCT, provided no LA GVHD or chronic GVHD had yet occurred. The goal was to characterize incidence, characteristics, and outcome of late post-HCT immune-mediated disorders (IMDs): LA GVHD, chronic GVHD, cutaneous sclerosis, and bronchiolitis obliterans. Classic acute GVHD (occurring within 100 days post-HCT) was not considered an IMD. Excluded were those with anticipated survival <6 months, relapse, autoimmune disorder within 5 years, or inherited immunodeficiency. Clinical data and research blood and urine samples were collected at baseline and then at day 180 or 365 post-HCT. Additional data and samples were collected at time of LA GVHD or chronic GVHD onset and then 3 or 6 months later. Subsequent clinical follow-up occurred annually.
LA GVHD grading and treatment
The current analysis includes 83 patients in whom LA GVHD developed as the first IMD post-HCT.12 Diagnosis of LA and chronic GVHD was made per National Institutes of Health (NIH) consensus guidelines.1 LA GVHD was classified as recurrent if there was a recurrence of acute GVHD after day 100 or de novo if acute GVHD presented after day 100 for the first time. Persistent LA GVHD was not captured in the study, as it was a continuation of prior acute GVHD. LA GVHD was diagnosed as a clinical syndrome and supported by biopsy of involved organs when deemed necessary. Grading for LA GVHD used criteria for classic acute GVHD.13 Those with suspected liver involvement due to transaminitis but without bilirubin elevation (n = 34) were not included for LA GVHD grading but were included for the analyses of outcomes. As no difference in overall survival (OS) was seen according to presence or type of liver involvement vs other LA GVHD, and exploratory analyses did not support significant differences in outcome according to degree of transaminase or alkaline phosphatase elevation, we could not pursue focused questions on liver staging in LA GVHD further.
Treatment decisions were not controlled. Beyond first-line therapy, we considered the following significant additional systemic immunosuppressive therapy (IST): (1) addition of new systemic steroid therapy, (2) steroid increase from 1 mg/kg prednisone per day to 2 mg/kg prednisone per day (or equivalent) due to progressive GVHD, and (3) addition of a different systemic IST.
Outcomes
Clinical response to therapy at days 28, 56, and 180 was scored as follows: complete response (CR), complete resolution of GVHD in all organs; partial response (PR), improvement in GVHD stage in at least 1 GVHD organ without worsening in others; additional systemic IST, or worsening of GVHD by at least 1 stage increment in ≥1 organ constituted progressive disease (PD); or stable disease (all other responses).
OS was defined as time from diagnosis of LA GVHD to death or last follow-up. Failure-free survival (FFS) was defined as absence of relapse, death, or additional systemic IST (given for any indication) beyond first-line therapy.14
Morbidity assessments included NIH Common Terminology Criteria for Adverse Events grade 2 or higher infections and hospital admissions within 6 months after the diagnosis of LA GVHD.
Statistical analysis
Time to onset of LA GVHD was calculated from HCT, with patients censored at last contact date. Chronic GVHD, relapse, and death were treated as competing risks. Cumulative incidence is reported at 2 years after HCT.15
For those with LA GVHD, patient characteristics at HCT are presented and compared between recurrent and de novo subtypes. The Wilcoxon rank sum test was performed for continuous variables and Fisher’s exact test for categorical variables. Organ involvement, stage, overall grade, treatment, and response rates are reported. OS and FFS were calculated from LA GVHD diagnosis, with patients censored at last contact. Time to complete IST discontinuation (defined as complete removal of all systemic IST without need to initiate again for subsequent GVHD) was calculated, with patients censored at last contact date. Cox regression models were used to identify risk factors for OS, FFS, and time to discontinuation of IST. These included study site, gender, race/ethnicity, age, type of onset, organ involved, maximum stage, disease diagnosis, disease status, conditioning regimen, patient/donor cytomegalovirus status, prior classic acute GVHD, donor/patient gender combination, and donor match. In a separate model, we examined mortality from the time of HCT, with LA GVHD and chronic GVHD included as time-varying covariates. Statistical analyses were performed with SAS/STAT software, version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Analysis of circulating angiogenic factors
Plasma levels of EGF receptor (EGFR) ligands (EGF, AREG, and HB-EGF), EGFR ligand sheddases (ADAM10 and ADAM17), and regulators of angiogenesis (VEGF-A, follistatin, and activin A) were determined in 55 patients with LA GVHD from the Chronic GVHD Consortium who had onset samples available for study. Because the EGFR ligands bind EGFR with different affinities, we also tested ratios of EGF (the strongest ligand) with weaker ligands (HB-EGF and AREG),16 hypothesizing that higher proportions of weaker ligands may be associated with poor outcomes. Cryopreserved plasma samples banked according to protocol were shipped frozen on dry ice to the Cytokine Reference Laboratory at the University of Minnesota. Samples had not undergone any prior freeze-thaw cycles. Measurements were log-transformed, and those smaller than the lower limit of quantification were imputed as the lower limit of quantification. Ratio Estimate and P values were determined from a multivariable linear model adjusting for prior acute GVHD, month from transplant to sample draw, study site, patient age at sample draw, and donor match category. The ratio estimate of each marker represents the estimated ratio of GVHD over controls. EGF, HB-EGF, follistatin, and VEGF-A were determined by multiplex bead array (MilliPLEX; Millipore, Billerica MA). AREG and activin A were determined by enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis MN). ADAM10 and ADAM17 were determined by enzyme-linked immunosorbent assay (MyBioSource, Inc., San Diego, CA). We compared clinical characteristics of patients with vs without available samples from this Chronic GVHD Consortium cohort and found no difference (see supplemental Table 1, available on the Blood Web site). Data on prior classic acute and systemic IST at sample collection are reported (supplemental Tables 2 and 3, respectively). These 55 samples from patients with LA GVHD (cohort 1) were collected within a median of 7 days prior to onset and compared with samples from 50 patients without LA GVHD (controls) matched for age, donor match, study site, month from HCT to sample, and prior acute GVHD. Initial results indicated significant differences in AREG and AREG/EGF ratio in LA GVHD compared with controls. We then analyzed levels of these factors in an independent cohort of patients with LA GVHD from the Mount Sinai Acute GVHD International Consortium (MAGIC; cohort 2, n = 37) samples collected within a median of 16 days prior to onset (median 103 days post-HCT; interquartile range [IQR], 95-122) for validation. Levels of AREG, EGF, and AREG/EGF ratio from patients in both cohort 1 and cohort 2 were compared with controls using 2-sample t tests on log-transformed values. For determination of association of AREG and AREG/EGF ratios with organ severity, day 28 response to therapy, cause of death, OS, and nonrelapse mortality (NRM) in each cohort, the median levels of AREG and AREG/EGF ratio (20.5 pg/mL and 0.6, respectively) from cohorts 1 and 2 combined (excluding controls) were used.
To further determine patterns of AREG and AREG/EGF ratio with classic acute and chronic GVHD, we analyzed these levels in additional independent cohorts; the acute GVHD case and control samples were obtained from both MAGIC and Oregon Health & Science University (OHSU). Samples from classic acute GVHD cases (n = 24) were collected at a median of 28 days (IQR, 21-56). Acute GVHD control samples (n = 26) were collected at a median of 28 days post-HCT (IQR, 22-82) in patients who had no prior GVHD or relapse at the time of sample collection. Samples from patients with chronic GVHD (n = 26) and controls (n = 26) were obtained from the parent Chronic GVHD Consortium cohort study; these were matched by age, donor match, study site, month from HCT to sample, and prior acute GVHD.
Results
Patient characteristics
Of 909 patients in the Chronic GVHD Consortium cohort study, LA GVHD developed in 83, with a cumulative incidence of 6% at 6 months and 11% at 2 years after HCT. For these 83 patients, median time from HCT to enrollment in the parent cohort study was 83 days (range, −57 to 118). Median age of the LA GVHD cohort was 53 years (range, 19-73 years). Fifty-six percent of transplants were for acute leukemias, 71% received unrelated transplants, and 45% received myeloablative conditioning. Peripheral blood was used in 87% patients, and 6% received antithymocyte globulin or alemtuzumab. Baseline characteristics are shown in Table 1. Patients with recurrent LA GVHD were younger and had more myeloablative conditioning compared de novo LA GVHD. Median follow-up for survivors after LA GVHD diagnosis was 10.1 (range, 0.4-25.9) months.
Baseline characteristics of LA GVHD patients from the Chronic GVHD Consortium (cohort 1)
| Characteristic . | Recurrent (n = 48) . | De novo (n = 35) . | P value . |
|---|---|---|---|
| Age (y) at transplant; median (range) | 49.7 (19.3-71.4) | 59.5 (22.2-73.0) | .01 |
| Gender | .87 | ||
| Female | 17 (35%) | 13 (37%) | |
| Male | 31 (65%) | 22 (63%) | |
| Diagnosis | .80 | ||
| Chronic leukemia | 2 (4%) | 2 (6%) | |
| Acute leukemia | 28 (58%) | 18 (51%) | |
| MDS | 8 (17%) | 4 (11%) | |
| HD/NHL | 3 (6%) | 4 (11%) | |
| Other | 7 (15%) | 7 (20%) | |
| Disease risk | .87 | ||
| Early | 26 (54%) | 17 (49%) | |
| Intermediate | 18 (38%) | 15 (43%) | |
| Advanced | 4 (8%) | 3 (9%) | |
| Donor | .24 | ||
| Matched related | 10 (21%) | 13 (37%) | |
| Matched unrelated | 22 (46%) | 14 (40%) | |
| Mismatched | 15 (33%) | 8 (23%) | |
| Conditioning | .01 | ||
| Myeloablative | 27 (56%) | 10 (29%) | |
| Reduced intensity | 21 (44%) | 25 (71%) | |
| Stem cell source | .57 | ||
| BM | 2 (4%) | 2 (6%) | |
| PBSC | 40 (83%) | 31 (89%) | |
| Umbilical cord blood | 6 (13%) | 2 (6%) | |
| GVHD prophylaxis | |||
| Tac + Mtx | 7 (25%) | 29 (53%) | .02 |
| Tac + MMF | 7 (25%) | 11 (20%) | .60 |
| Csp + Mtx | 1 (4%) | 1 (2%) | .62 |
| Csp and MMF | 10 (36%) | 8 (15%) | .03 |
| Use of ATG or alemtuzumab | 1 (4%) | 3 (5%) | .70 |
| CMV serostatus | .42 | ||
| D− and R− | 16 (34%) | 9 (26%) | |
| D+ or R+ | 31 (66%) | 26 (74%) |
| Characteristic . | Recurrent (n = 48) . | De novo (n = 35) . | P value . |
|---|---|---|---|
| Age (y) at transplant; median (range) | 49.7 (19.3-71.4) | 59.5 (22.2-73.0) | .01 |
| Gender | .87 | ||
| Female | 17 (35%) | 13 (37%) | |
| Male | 31 (65%) | 22 (63%) | |
| Diagnosis | .80 | ||
| Chronic leukemia | 2 (4%) | 2 (6%) | |
| Acute leukemia | 28 (58%) | 18 (51%) | |
| MDS | 8 (17%) | 4 (11%) | |
| HD/NHL | 3 (6%) | 4 (11%) | |
| Other | 7 (15%) | 7 (20%) | |
| Disease risk | .87 | ||
| Early | 26 (54%) | 17 (49%) | |
| Intermediate | 18 (38%) | 15 (43%) | |
| Advanced | 4 (8%) | 3 (9%) | |
| Donor | .24 | ||
| Matched related | 10 (21%) | 13 (37%) | |
| Matched unrelated | 22 (46%) | 14 (40%) | |
| Mismatched | 15 (33%) | 8 (23%) | |
| Conditioning | .01 | ||
| Myeloablative | 27 (56%) | 10 (29%) | |
| Reduced intensity | 21 (44%) | 25 (71%) | |
| Stem cell source | .57 | ||
| BM | 2 (4%) | 2 (6%) | |
| PBSC | 40 (83%) | 31 (89%) | |
| Umbilical cord blood | 6 (13%) | 2 (6%) | |
| GVHD prophylaxis | |||
| Tac + Mtx | 7 (25%) | 29 (53%) | .02 |
| Tac + MMF | 7 (25%) | 11 (20%) | .60 |
| Csp + Mtx | 1 (4%) | 1 (2%) | .62 |
| Csp and MMF | 10 (36%) | 8 (15%) | .03 |
| Use of ATG or alemtuzumab | 1 (4%) | 3 (5%) | .70 |
| CMV serostatus | .42 | ||
| D− and R− | 16 (34%) | 9 (26%) | |
| D+ or R+ | 31 (66%) | 26 (74%) |
ATG, antithymocyte globulin; BM, bone marrow; CMV, cytomegalovirus; Csp, cyclosporine; D−/R−, donor negative/recipient negative; HD/NHL, Hodgkin’s disease/non-Hodgkin’s lymphoma; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; Mtx, methotrexate; PBSC, peripheral blood stem cells; Tac, tacrolimus.
LA GVHD characteristics
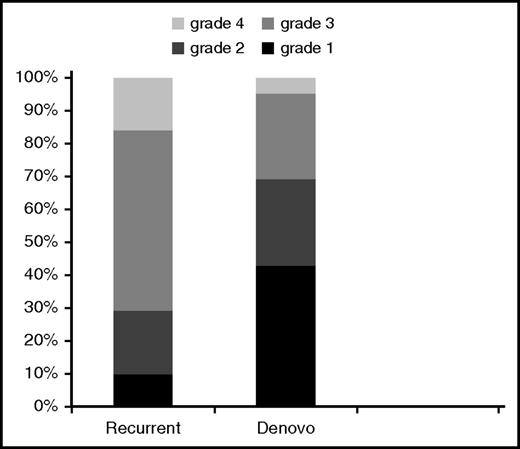
Diagnosis was made according to standard criteria,13 with 61% of patients with biopsy confirmation, at a median onset of 160 (IQR, 128-204) days after HCT. Single organ involvement at diagnosis was seen in 57 patients (skin 37%, liver 14%, and gut 49%), while 26 patients (31%) had involvement of ≥2 organs. Recurrent LA GVHD had greater overall grade (P = .006) compared with de novo GVHD (Figure 1).
Overall grade at LA GVHD onset. Comparison of recurrent vs de novo disease.
Of the 44 patients with liver GVHD, most (n = 34) had only transaminase and/or alkaline phosphatase elevation. Alkaline phosphatase was elevated in 11 out of 34 patients. Alanine aminotransferase was elevated in 24 out of 34 patients; 6 patients had alanine aminotransferase levels >5 times the upper limit of normal. Among the 44 cases, 8 had liver biopsy–confirmed GVHD, and of these, 2 also had concurrent gastrointestinal (GI) biopsies. Of the 36 without liver biopsy specimens, 7 had skin biopsies and 10 had GI biopsies confirming GVHD concurrently in these organs.
Treatment
Although primary therapy of LA GVHD was not mandated, interventions recapitulated standard primary acute GVHD therapy. Systemic steroids were newly started or increased in 67% of cases, and optimization of calcineurin inhibitor (new agent 7%, dose increased to achieve therapeutic levels 25%, continued therapy 30%) was performed. Topical (skin, GI) steroid agents were used in 44% of cases. Median dose of systemic corticosteroids used for initial treatment of LA GVHD was 1 mg/kg (range, 0.01-2 mg/kg). Additional systemic IST beyond first line was added for 28% of patients within 28 days, 11% of patients between 28 and 56 days, and 13% of patients between 56 and 180 days. In the majority of cases, new systemic IST was added for PD (87%, 89%, and 82% of cases, respectively, for each response interval); in the remaining, therapy was added for inadequate response or flare upon taper of initial therapy. The 3 most common interventions after initial treatment included adding or increasing steroid treatment (24%), extracorporeal photopheresis (11%), and sirolimus (5%). The median number of lines of therapy beyond first line for the total duration of follow-up was 1 (range, 0-4). Only 25% of patients discontinued IST by last follow-up at a median of 15.7 months (range 4-20 months) after LA GVHD onset.
Outcomes
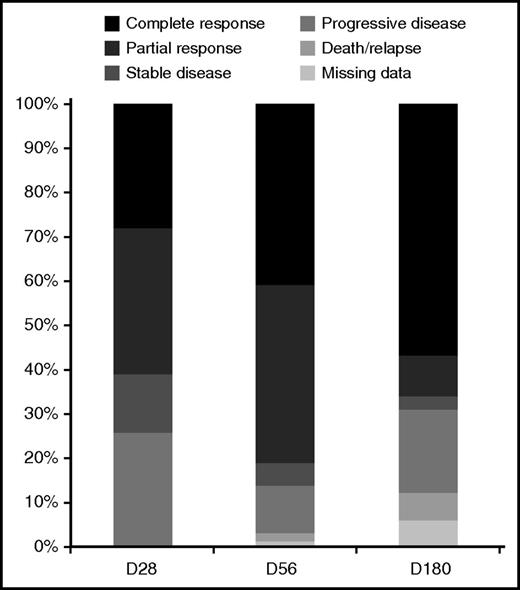
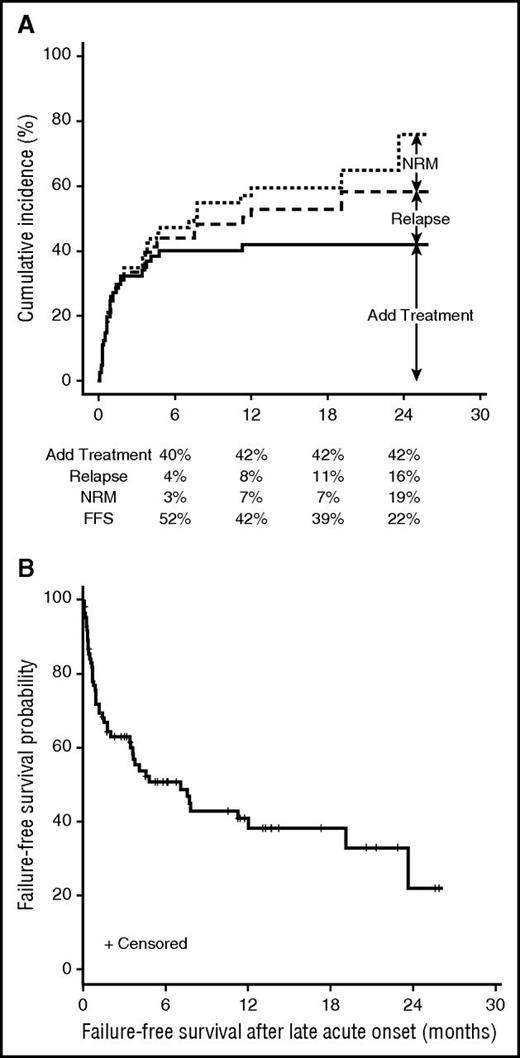
Of evaluable patients, 51 out of 83 (61%) had a complete or partial response (CR/PR) at 28 days and 54 out of 73 (74%) had a CR/PR by 180 days. Figure 2 shows the response at 28, 56, and 180 days. Additional outcomes are summarized in Table 2. There were 17 deaths; the main causes of death were GVHD, infection, or multiorgan failure in 70% cases and relapse in the remaining cases. Figure 3A shows the cumulative incidence and reasons for treatment failure. Median FFS was 7.1 months (95% confidence interval [CI], 3.4-19.1; Figure 3B). The estimated OS was 56% at 2 years. No patient-, transplant-, or GVHD-related factors, including type of onset of LA GVHD, emerged as significant predictors for OS, FFS, or discontinuing IST.
Outcomes after LA GVHD diagnosis from the Chronic GVHD Consortium (cohort 1)
| Event . | Frequency n (%)* . | Median time to event in months from LA GVHD diagnosis (range) . |
|---|---|---|
| Death | 17 (20) | 5.8 (0.8-23.6) |
| Relapse | 8 (10) | 7.7 (0.7-19.1) |
| Development of chronic GVHD | 23 (28) | 5.4 (0.8-11.3) |
| Recurrence of LA GVHD after complete resolution | 23 (28) | 7.0 (0-13) |
| Successful discontinuation of IST | 20 (24) | 15.7 (4-20) |
| Event . | Frequency n (%)* . | Median time to event in months from LA GVHD diagnosis (range) . |
|---|---|---|
| Death | 17 (20) | 5.8 (0.8-23.6) |
| Relapse | 8 (10) | 7.7 (0.7-19.1) |
| Development of chronic GVHD | 23 (28) | 5.4 (0.8-11.3) |
| Recurrence of LA GVHD after complete resolution | 23 (28) | 7.0 (0-13) |
| Successful discontinuation of IST | 20 (24) | 15.7 (4-20) |
Reported frequencies in this table are raw percentages, not cumulative incidence.
Clinical outcomes of LA GVHD. (A) Cumulative incidence of treatment failure after initial systemic treatment. (B) Failure-free survival after onset of LA GVHD.
Clinical outcomes of LA GVHD. (A) Cumulative incidence of treatment failure after initial systemic treatment. (B) Failure-free survival after onset of LA GVHD.
In a different model using the entire cohort of 909 patients, OS and NRM were evaluated for association with GVHD as time-dependent covariates. Development of LA GVHD was associated with increased overall mortality (hazard ratio [HR], 1.70; 95% CI, 1.01-2.86; P = .05) and increased NRM (HR, 1.75; 95% CI, 0.96-3.18; P = .07). In contrast, chronic GVHD was not associated with increased mortality (HR, 0.74; 95% CI, 0.49-1.13; P = .16) or NRM (HR, 0.84; 95% CI, 0.52-1.35; P = .46).
Morbidity
Grade 2 or higher infections occurred in 40% of patients, with an average of 2.3 infectious episodes during the 6-month follow-up period. The median number of hospital days in the first 6 months after the diagnosis of LA GVHD was 15 days (IQR, 5-47 days). The main causes of hospitalization included GVHD (37%) and/or infections (37%).
Analyses of circulating angiogenic factors
Because follistatin has been associated with survival in classic acute GVHD,9 we first sought to ascertain its association in patients with LA GVHD from the Chronic GVHD Consortium (cohort 1). Patients with LA GVHD and high follistatin levels (>2000 pg/mL) in this study had a HR for OS of 2.32 (P = .22) and an NRM of 1.88 (P = .38). There was no significant correlation of follistatin with activin A levels (Spearman’s ρ = −0.05, P = .6), suggesting that elevated follistatin levels may not be solely driven by activin A.
Plasma levels of AREG were more than double in cohort 1 compared with controls (ratio estimate [estimated ratio of GVHD over controls] = 2.5; 95% CI, 1.6-3.9; P < .001; Table 3; Figures 4 and 5A). The area under the curve for AREG as a single marker of LA GVHD was 0.68. Plasma AREG was elevated in de novo (ratio estimate, 2.72; P < .001) and recurrent (ratio estimate = 2.15, P = .01) LA GVHD compared with controls; no significant difference was observed between de novo and recurrent cases. Results were similar after adjustment for prednisone use at the time of the sample (P < .001), and prednisone use was not associated with AREG (P = .8). AREG did not correlate with acute GVHD III-IV severity (P = .5), day 28 response to therapy (P = .2), OS (HR 1.99, P = .26) or NRM (HR 2.33, P = .22). Mean AREG levels were nearly identical in cohort 1 and cohort 2 (65 pg/mL and 65.9 pg/mL, respectively, vs 19.9 pg/mL in controls; ratio estimate, 1.7; P = .08; Table 3; Figure 5A), although the median AREG level in cohort 2 was lower. Unlike cohort 1, patients with maximal grade III-IV GVHD (n = 6) had higher AREG levels than those with grade I-II (median 118 pg/mL vs 12.5 pg/mL, P = .02). However, AREG levels alone were not associated with OS (HR=2.41, P = .11) or NRM (HR 1.79, P = .19), as was the case for cohort 1. The majority (33 of 37 patients) had a CR at 4 weeks after LA GVHD onset, thus we were unable to assess for an association of angiogenic factors and response in cohort 2.
Comparison of angiogenic factors in patients with LA GVHD (inclusive of recurrent and de novo LA GVHD), classic acute GVHD, and chronic GVHD vs their respective controls
| Variable . | LA GVHD cohort 1 (n = 55) . | LA GVHD controls (n = 50) . | P value . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 65.0 | 143.5 | 24.0 | 4.0 | 843.2 | 19.9 | 18.1 | 16.6 | 0.7 | 84.0 | <.001 |
| EGF | 53.7 | 52.2 | 38.0 | 0.9 | 215.6 | 52.0 | 46.6 | 47.8 | 0.9 | 216.4 | .2 |
| AREG/EGF ratio | 11.8 | 42.0 | 0.82 | 0.07 | 289.3 | 1.6 | 4.8 | 0.42 | 0.01 | 25.9 | <.001 |
| Variable . | LA GVHD cohort 1 (n = 55) . | LA GVHD controls (n = 50) . | P value . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 65.0 | 143.5 | 24.0 | 4.0 | 843.2 | 19.9 | 18.1 | 16.6 | 0.7 | 84.0 | <.001 |
| EGF | 53.7 | 52.2 | 38.0 | 0.9 | 215.6 | 52.0 | 46.6 | 47.8 | 0.9 | 216.4 | .2 |
| AREG/EGF ratio | 11.8 | 42.0 | 0.82 | 0.07 | 289.3 | 1.6 | 4.8 | 0.42 | 0.01 | 25.9 | <.001 |
Both LA GVHD cohorts 1 (n = 55) and 2 (n = 37) were compared with LA GVHD controls (n = 50) via 2-sample t test of log-transformed values. Cohort 1, LA GVHD vs matched control comparison from national Chronic GVHD Consortium; cohort 2, validation cohort of patients with LA GVHD at onset from the Mount Sinai Acute GVHD International Consortium (MAGIC). Acute GVHD, classic acute GVHD vs matched controls from MAGIC and Oregon Health & Science University. Chronic GVHD, chronic GVHD and matched control samples from Chronic GVHD Consortium. Bold indicates statistical significance.
SD, standard deviation.
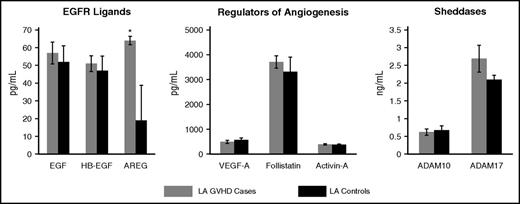
Plasma levels of angiogenic factors in patients with LA GVHD and their respective controls. Data are presented as mean ± standard error of the mean. Asterisk indicates statistically significant difference in amphiregulin between LA GVHD cases and controls.
Plasma levels of angiogenic factors in patients with LA GVHD and their respective controls. Data are presented as mean ± standard error of the mean. Asterisk indicates statistically significant difference in amphiregulin between LA GVHD cases and controls.
AREG, AREG/EGF ratio, OS, and NRM after LA GVHD. Boxplot of (A) AREG and (B) AREG/EGF ratio in LA GVHD cases vs controls. (C) OS and (D) NRM (d) by AREG/EGF ratio. *Cohort 1, LA GVHD vs matched control comparison from national Chronic GVHD Consortium; cohort 2, validation cohort of patients with LA GVHD at onset from the Mount Sinai Acute GVHD International Consortium (MAGIC).
AREG, AREG/EGF ratio, OS, and NRM after LA GVHD. Boxplot of (A) AREG and (B) AREG/EGF ratio in LA GVHD cases vs controls. (C) OS and (D) NRM (d) by AREG/EGF ratio. *Cohort 1, LA GVHD vs matched control comparison from national Chronic GVHD Consortium; cohort 2, validation cohort of patients with LA GVHD at onset from the Mount Sinai Acute GVHD International Consortium (MAGIC).
Because both AREG and EGF signal through EGFR, we determined whether the ratio of these EGFR ligands would provide additional diagnostic/prognostic utility. In cohort 1, the AREG/EGF ratio (ratio estimate, 3.6; 95% CI, 1.80-7.3; P = .001; Figure 5B) was more strongly associated with LA GVHD compared with AREG alone. AREG/EGF was elevated in both de novo (ratio estimate, 4.37; P < .001) and recurrent (ratio estimate, 2.59; P = .05) LA GVHD compared with controls; no significant difference was observed between de novo and recurrent cases. Results were unchanged after adjustment for prednisone use (P < .001). The area under the curve of AREG/EGF was similar to AREG alone at 0.67. However, the AREG/EGF ratio was even more strongly associated with survival, such that ratios greater than or equal to the median conferred an HR of 9.4 (95% CI, 1.2-72.8) for OS (Figure 5C) and an HR of 7.8 (95% CI, 1.0-61.9 for NRM; Figure 5D). Causes of death are shown in supplemental Table 4. There were no significant associations of AREG/EGF ratio and organ severity (P = .5), day 28 (P = .3), or day 56 (P = .3) response to therapy in cohort 1, although the highest median AREG/EGF ratio was observed in patients who died by day 180 (ratio 3.8, compared with 0.6 for CR, 0.6 for PR, 0.06 for stable disease, and 0.5 for PD; P = .047). There was no association between HB-EGF/EGF and LA GVHD diagnosis (P = .68) or survival (P = .44). We next sought to verify our findings for the association of AREG/EGF ratios with outcomes in the validation cohort from MAGIC (cohort 2; supplemental Table 5). Similar to cohort 1, the ratio of AREG/EGF was significantly elevated in cohort 2 cases compared with the controls (ratio estimate, 2.7; P = .02). We found that the median AREG/EGF ratio was a significant predictor for OS (HR, 6.2; 95% CI, 2.2-17.8; Figure 5C) and NRM (HR, 13.5; 95% CI, 2.9-63.5; Figure 5D) in cohort 2. While we acknowledge that additional work to define an optimal threshold value for AREG/EGF ratio may improve biomarker performance, classification based on the median value for AREG/EGF was strongly predictive of OS and NRM in both cohort 1 and 2.
AREG levels (15.9 pg/mL vs 8.6 pg/mL; P = .002) and AREG/EGF ratios (10.8 vs 2.9; P = .2) were higher in classic acute GVHD patients compared with controls, suggesting similarity between classic and LA GVHD. Adjustment for source of samples (MAGIC vs OHSU) did not alter the results of this comparison; the association of AREG with acute GVHD was statistically significant even when the sources were analyzed separately (MAGIC, P = .02; OHSU, P = .01). However, AREG levels (15.8 vs 14.3 pg/mL; P = .6) and the AREG/EGF ratio (0.4 vs 0.2; P = .2) were not significantly different in chronic GVHD patients compared with controls (Tables 3, 4, 5, and 6).
Comparison of angiogenic factors in patients with LA GVHD (inclusive of recurrent and de novo LA GVHD), classic acute GVHD, and chronic GVHD vs their respective controls
| Variable . | LA GVHD Validation cohort 2 (n = 37) . | P value . | ||||
|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 65.9 | 152.2 | 14.4 | 2.5 | 729.1 | .08 |
| EGF | 46.1 | 61.0 | 21.6 | 0.9 | 239.0 | .1 |
| AREG/EGF ratio | 25.2 | 132.6 | 0.48 | 0.06 | 809.0 | .02 |
| Variable . | LA GVHD Validation cohort 2 (n = 37) . | P value . | ||||
|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 65.9 | 152.2 | 14.4 | 2.5 | 729.1 | .08 |
| EGF | 46.1 | 61.0 | 21.6 | 0.9 | 239.0 | .1 |
| AREG/EGF ratio | 25.2 | 132.6 | 0.48 | 0.06 | 809.0 | .02 |
Both LA GVHD cohorts 1 (n = 55) and 2 (n = 37) were compared with LA GVHD controls (n = 50) via 2-sample t test of log-transformed values. Cohort 1, LA GVHD vs matched control comparison from national Chronic GVHD Consortium; cohort 2, validation cohort of patients with LA GVHD at onset from the Mount Sinai Acute GVHD International Consortium (MAGIC). Acute GVHD, classic acute GVHD vs matched controls from MAGIC and Oregon Health & Science University. Chronic GVHD, chronic GVHD and matched control samples from Chronic GVHD Consortium. Bold indicates statistical significance.
SD, standard deviation.
Comparison of angiogenic factors in patients with LA GVHD (inclusive of recurrent and de novo LA GVHD), classic acute GVHD, and chronic GVHD vs their respective controls
| Variable . | Acute GVHD (n = 24) . | Acute GVHD controls (n = 26) . | P value . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 100.8 | 184.1 | 15.9 | 4.2 | 753.6 | 17.9 | 40.6 | 8.6 | 1.3 | 213.0 | .002 |
| EGF | 28.5 | 48.1 | 0.9 | 0.9 | 165.3 | 17.1 | 27.7 | 0.9 | 0.9 | 99.6 | .8 |
| AREG/EGF ratio | 90.4 | 195.6 | 10.8 | 0.04 | 837.3 | 15.9 | 46.2 | 2.9 | 0.01 | 236.7 | .2 |
| Variable . | Acute GVHD (n = 24) . | Acute GVHD controls (n = 26) . | P value . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 100.8 | 184.1 | 15.9 | 4.2 | 753.6 | 17.9 | 40.6 | 8.6 | 1.3 | 213.0 | .002 |
| EGF | 28.5 | 48.1 | 0.9 | 0.9 | 165.3 | 17.1 | 27.7 | 0.9 | 0.9 | 99.6 | .8 |
| AREG/EGF ratio | 90.4 | 195.6 | 10.8 | 0.04 | 837.3 | 15.9 | 46.2 | 2.9 | 0.01 | 236.7 | .2 |
Both LA GVHD cohorts 1 (n = 55) and 2 (n = 37) were compared with LA GVHD controls (n = 50) via 2-sample t test of log-transformed values. Cohort 1, LA GVHD vs matched control comparison from national Chronic GVHD Consortium; cohort 2, validation cohort of patients with LA GVHD at onset from the Mount Sinai Acute GVHD International Consortium (MAGIC). Acute GVHD, classic acute GVHD vs matched controls from MAGIC and Oregon Health & Science University. Chronic GVHD, chronic GVHD and matched control samples from Chronic GVHD Consortium. Bold indicates statistical significance.
SD, standard deviation.
Comparison of angiogenic factors in patients with LA GVHD (inclusive of recurrent and de novo LA GVHD), classic acute GVHD, and chronic GVHD vs their respective controls
| Variable . | Chronic GVHD (n = 26) . | Chronic GVHD controls (n = 26) . | P value . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 41.5 | 76.5 | 15.8 | 1.3 | 382.8 | 25.2 | 27.4 | 14.3 | 1.3 | 102.1 | .6 |
| EGF | 102.3 | 206.5 | 41.7 | 0.9 | 984.0 | 90.2 | 113.0 | 54.8 | 4.01 | 559.8 | .2 |
| AREG/EGF ratio | 6.4 | 16.0 | 0.4 | 0.01 | 53.8 | 0.7 | 1.01 | 0.2 | 0.02 | 3.6 | .2 |
| Variable . | Chronic GVHD (n = 26) . | Chronic GVHD controls (n = 26) . | P value . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | Median . | Min . | Max . | Mean . | SD . | Median . | Min . | Max . | ||
| AREG | 41.5 | 76.5 | 15.8 | 1.3 | 382.8 | 25.2 | 27.4 | 14.3 | 1.3 | 102.1 | .6 |
| EGF | 102.3 | 206.5 | 41.7 | 0.9 | 984.0 | 90.2 | 113.0 | 54.8 | 4.01 | 559.8 | .2 |
| AREG/EGF ratio | 6.4 | 16.0 | 0.4 | 0.01 | 53.8 | 0.7 | 1.01 | 0.2 | 0.02 | 3.6 | .2 |
Both LA GVHD cohorts 1 (n = 55) and 2 (n = 37) were compared with LA GVHD controls (n = 50) via 2-sample t test of log-transformed values. Cohort 1, LA GVHD vs matched control comparison from national Chronic GVHD Consortium; cohort 2, validation cohort of patients with LA GVHD at onset from the Mount Sinai Acute GVHD International Consortium (MAGIC). Acute GVHD, classic acute GVHD vs matched controls from MAGIC and Oregon Health & Science University. Chronic GVHD, chronic GVHD and matched control samples from Chronic GVHD Consortium.
SD, standard deviation.
Discussion
Classification of GVHD according to clinical manifestations rather than time post-HCT was proposed by the NIH consensus conference in 2005 and revised recently.1,17 Here, we present a prospective evaluation of LA GVHD. Wide variation exists in prior estimates of incidence of LA GVHD.2-4,18-21 Our data support an incidence of 11% at 2 years after HCT. De novo onset LA GVHD is more common in patients undergoing reduced intensity HCT. Single- and multiple-organ involvement is common, and hepatic involvement frequently occurs in the absence of bilirubin elevation.
Based on current practices, a minority of LA GVHD patients have durable success. Response to initial therapy recapitulates that reported in classic acute GVHD,22 yet use of second-line IST is common. Freedom from GVHD is infrequent, with recurrent LA GVHD among responders (28%), and development of chronic GVHD (37%, higher than 20% to 28% reported in prior literature).3,19 By 6 months only 24% will successfully discontinue all IST, and 6 month FFS (52% after LA GVHD) is lower than chronic GVHD (68%).23 Although prior reports are conflicting,3,13-15 our prospective data support that LA GVHD is associated with an inferior prognosis. We observed no survival differences between recurrent vs de novo LA GVHD.2,7
Classic acute GVHD is a well-known predictor of resource utilization especially during the first 100 days of HCT.24-26 We found that LA GVHD may contribute to costs after 100 days of HCT as indicated by a median of 15 hospital days in the 6 months after diagnosis of LA GVHD. This is partly due to morbidity from ongoing IST and related complications.27
This study demonstrated that altered proportions of EGFR ligands, specifically AREG and EGF, are of diagnostic and prognostic significance for LA GVHD. It is notable that mean levels of EGF in LA cases (approximately 50 pg/mL) were similar to LA controls and nearly 3-fold higher than those previously observed in our prior study of classic acute GVHD.9 These differences in EGF levels may reflect the longer time post-HCT or biological differences between classic and LA GVHD.
We also found that circulating AREG is markedly elevated in both classic and LA GVHD. An elevated AREG/EGF ratio is significantly associated with inferior outcomes after the diagnosis of LA GVHD. We validated this finding in an independent cohort, a major strength of the study. Like EGF, AREG signals through EGFR, although it is a lower-affinity ligand that exhibits partial agonist behavior.28 AREG, which is induced predominantly in settings associated with type 2 inflammation,29 is known for its tissue repair function; a recent report identified AREG as a regulatory T-cell–derived factor required for control of tissue damage.30 It is possible that elevated AREG levels in both classic and LA GVHD reflect more extensive tissue damage than can be appreciated by clinical staging alone. We hypothesize that excess AREG, a weak EGFR agonist relative to EGF, may compete for receptor binding and result in diminished wound healing, although this will require further investigation. Like AREG, EGF also has wound-healing and immune regulatory roles. EGF has an anti-inflammatory role by downregulating tissue expression of chemokines such as CCL2, CXCL10, and CCL25,31 and elevated AREG relative to EGF may have distinct immunologic effects. Our observation of an elevated plasma AREG level and AREG/EGF ratio offers novel insight into response to both classic and LA GVHD and differentiates the syndromes from chronic GVHD. Additional studies are needed to determine the mechanisms underlying our observations.
Further studies are needed to delineate the cellular sources of these EGFR ligands and mechanisms underlying their contribution to clinical outcomes. It is possible that these results may also translate to developing novel therapies for LA GVHD by focusing on controlling AREG-associated type 2 inflammation, identifying ways to augment EGF expression to correct the imbalance of EGFR ligands, or both.
Our analysis has several limitations. First, the small number of LA GVHD patients limited our ability to perform a multivariate analysis. Second, LA GVHD may have been precipitated by IST taper, and taper practices were both not standardized and not adequately captured in this cohort study. Some evidence suggests that intentionally prolonged IST may be beneficial.32 Next, we acknowledge that not all LA GVHD cases had biopsy confirmation but rather were judged to be LA GVHD per clinical manifestations. Varied primary therapy practices may have contributed to heterogeneity of secondary IST and outcomes. Finally, we acknowledge that larger-scale discovery work may identify both additional mechanistic insight into LA GVHD and provide useful diagnostic and prognostic information.
LA GVHD is a distinct syndrome associated with high morbidity, mortality, and poor FFS. AREG is elevated in LA GVHD, and the excess of plasma AREG relative to EGF appears to be a negative prognostic factor for OS and NRM. The results from this study help characterize this LA GVHD population and can therefore guide future mechanistic studies and provide a framework for development of future clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Michael Ehrhardt of the University of Minnesota Cytokine Reference Laboratory for technical assistance. The authors express their sincere appreciation for assistance from Rachel Young, project manager for the Mount Sinai Acute GVHD International Consortium.
This study was funded by National Institutes of Health, National Cancer Institute grants CA163438 and CA118953. The Chronic GVHD Consortium (U54 CA163438) is part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Disease Research (ORDR), NCATS, funded through collaboration between NCATS and the National Cancer Institute. The angiogenic factor studies were made possible by the Masonic Cancer Center, University of Minnesota.
Authorship
Contribution: N.K., S.G.H., S.J.L., and J.P. designed the study; N.K., S.G.H., H.D.L., and J.P. conducted the literature search and wrote the first draft of the manuscript. X.C. and B.S. did the statistical analyses; S.G.H. and A.P.-M. performed the circulating angiogenic factors analyses; and all other authors contributed patient plasma samples, assisted in interpretation of the findings, offered critical review of the paper, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shernan G. Holtan, University of Minnesota, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: sgholtan@umn.edu.
References
Author notes
S.G.H. and N.K. contributed equally to this study.