Abstract
Background: Autologous Stem Cell Transplantation (ASCT) is a standard of care for eligible NDMM pts. Current induction and consolidation regimen associate a proteasome inhibitor (PI), immunomodulatory drug (IMiD) and dexamethasone (Dex). Efforts to further improve outcomes are still needed, mainly by increasing the depth of tumor reduction and the duration of response. As response rate improves with time, we may need to extend cycles numbers. The benefit of bortezomib could be hampered by its neurological side effects. The IFM decided to evaluate, in the transplant setting, Carfilzomib (Carf), a non neurotoxic PI, with Lenalidomide (Len) and Dex as prolonged induction and consolidation regimen followed by Len maintenance.
Methods: This open-label, single arm, phase II study was conducted at 10 IFM transplant centers, with enrollment between 03-11/2014. Pts under 66 with symptomatic NDMM received four 28-day induction cycles of KRd= Carf 20/36mg/m2 (D1-2, 8-9, 15-16), Len 25 mg (D1-21), Dex 20 mg (D1-2, 8-9, 15-16, 22-23). Stem cell collection was planned for all pts after high dose (HD) cyclophosphamide. All pts proceeded to HD melphalan (200 mg/m2) followed by ASCT. Two months after hematological recovery, pts received four 28-day consolidation cycles of KRd (at last tolerated dose) followed by 1 year of Len maintenance (10 mg, D1-21). The primary objective was sCR rates at the completion of consolidation. Secondary objectives reported here included response rates after induction, ASCT and consolidation; PFS and safety profile of the KRd combination as induction and consolidation. Responses (Central Lab Dr Puissant, Toulouse) were assessed according to International Uniform Response Criteria. Flow cytometric analysis of plasma cells for Minimal Residual Disease MRD (Central Lab Dr Robillard, Nantes) was performed at each step of the program for pts at least in VGPR. For informative pts, MRD by Next Generation Sequencing NGS (Central Lab Pr Avet-Loiseau, Toulouse) was also analysed. Adverse events (AEs) were graded using the CTCAE v4.03.
Patients: Forty-eight pts with symptomatic MM were screened, 46 were enrolled and received at least 1 dose of treatment. Baseline characteristics of the treated pts were: median age = 56 years (range 40-65); ISS= 1 in 46%, 2 in 48% and 3 in 6% of pts. Adverse cytogenetics (17p deletion and/or t(4;14); central Lab Pr Avet-Loiseau, Toulouse) were observed in 9 of 43 (21%) assessable pts.
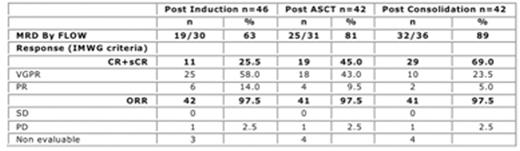
Results: All pts but 4 pts remain on study at data cut-off. Forty-three patients completed induction, 42 underwent ASCT, 41 completed consolidation and 27 patients are currently on maintenance phase. Considering efficacy, at the completion of consolidation, 23 sCR pts or more were required to claim activity of the program with prolonged KRd induction and consolidation. Among 42 evaluable pts, 27 were in sCR. Overall Response Rate (ORR) was 97.5%, including 23.5% VGPR, 69% CR or better and 32/36 pts (89%) were MRD negative by flow. For pts tested by NGS, 13/22 (59%) were MRD negative. CR or better rates improved at each step of the program. Description of responses is given in table 1. With a median follow-up of 20 months from start of therapy, 4 events were reported: 2 pts had progressive disease (PD) and 2 pts died without PD. Median PFS was not reached. Considering safety, there was no KRd related death, 4 pts permanently discontinued combined treatment due to AEs: 3 during induction (1 cardiac failure, 1 pneumonia, 1 jugular vein thrombosis ) and 1 post ASCT ( lethal septic choc). Overall, 44 serious AEs were reported in 30 pts (65%) including 8 cardiovascular events (2 cardiac failures, 1 bradycardia, 2 pulmonary embolisms and 3 thrombosis despite adequate prophylaxis). Overall, 20 cardiovascular events were reported: 7 cardiac disorders and 13 thrombosis. Other SAEs included infections in 12 pts (26%) and musculoskeletal disorders in 8 pts (17%). The 2 most common grade 3/4 AEs (>10%) related to KRd were hematological toxicities and infections, mainly post ASCT, during consolidation safety period. There was no grade 3/4 sensory peripheral neuropathy.
Conclusion: 8 cycles of KRd as induction and consolidation in the transplant setting produce high quality responses in NDMM pts. Safety profile seems acceptable but cardiovascular AEs if confirmed in other studies might impact results. Updated efficacy and safety data will be presented during the meeting including complete MRD analysis by NGS.
Roussel:BMS: Other: lecture fees; sanofi: Other: lecture fees; AMGEN: Consultancy, Other: lecture fees, Research Funding; celgene: Consultancy, Other: lecture fees, Research Funding; janssen: Consultancy, Other: lecture fees. Belhadj:janssen: Consultancy; novartis: Consultancy. Facon:celgene: Consultancy; janssen: Consultancy; sanofi: Consultancy; BMS: Consultancy; novartis: Consultancy; amgen: Consultancy. Garderet:Novartis: Consultancy; Takeda: Consultancy; Amgen: Consultancy; BMS: Consultancy, Honoraria. Fohrer:celgne: Consultancy; amgen: Consultancy. Moreau:Janssen: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria; Amgen: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Celgene: Honoraria. Leleu:TEVA: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; LeoPharma: Honoraria; Pierre Fabre: Honoraria; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Avet-Loiseau:celgene: Consultancy; amgen: Consultancy; sanofi: Consultancy; janssen: Consultancy. Attal:amgen: Consultancy, Research Funding; celgene: Consultancy, Research Funding; janssen: Consultancy, Research Funding; sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal