Abstract
Introduction: Mantle cell lymphoma (MCL) is a rare and incurable subtype of B-cell lymphoma. Intense chemo-immunotherapy with 8 cycles of Rituximab-HyperCVAD alternating with Rituximab-Methotrexate-Ara C is associated with an overall survival of 10.7 years but the 10-year cumulative incidence of therapy-related myeloid neoplasm was 6.2%. The ibrutinib-rituximab combination has produced durable responses in 88% of patients with relapsed and refractory MCL with acceptable toxicity. This gives rise to a "Window" of opportunity to use chemotherapy-free induction with ibrutinib plus rituximab followed by fewer cycles of chemo-immunotherapy consolidation in young and fit patients with newly-diagnosed, untreated MCL.
Methods: Enrolment began in June 2015 for a Phase II single-center clinical trial consisting of an initial chemotherapy-free phase (window) of ibrutinib and rituximab combination treatment in Part 1 until best response, followed by a shortened course of intense chemo-immunotherapy in Part 2 among young newly diagnosed MCL patients of ≤65 years. The primary objective was to evaluate the response rate of ibrutinib plus rituximab. The secondary objectives were to evaluate the progression free survival (PFS) of ibrutinib plus rituximab after consolidation with a shortened number of cycles of intense chemo-immunotherapy, and to further evaluate the toxicity profile. Ibrutinib is dosed at 560 mg orally, daily, continuously. Rituximab is dosed at 375 mg/m2 IV weekly x 4 during cycle 1 (28 days cycle), then day 1 of cycles 3-12. Intense chemo-immunotherapy consists of rituximab plus cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD); alternating every 28 days with rituximab plus high-dose methotrexate-Ara C. If in complete remission (CR) after initial ibrutinib and rituximab treatment, a total of 4 additional treatments of intense chemo-immunotherapy are given. If the patient is in partial response or progression, and if responding to intensive chemo-immunotherapy, a total of 2 cycles of chemo-immunotherapy therapy are administered beyond achievement of CR.
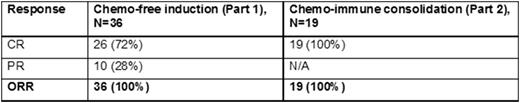
Results: As of August 2, 2016, we have completed the target enrolment by accruing 50 out 50 patients with newly-diagnosed untreated MCL. Forty one (n=41) patients have begun treatment and 36 are evaluable for response. Of the 36 evaluable patients, overall response rate (ORR) to Part 1 alone (Ibrutinib plus rituximab) is 100% (n=36) with PR in 28% (n=10) and CR in 72% (n=20). Nineteen 19 patients have completed both Part 1 (ibrutinib and rituximab) and Part 2 (chemo-immunotherapy). The ORR to both Part 1 and Part 2 (n=19) was 100% and was equal to the CR rate (100%, n=19), i.e. all have achieved a CR to Part 1 and Part 2. Toxicities are recorded as the number of patients experiencing a certain adverse event. Regardless of their relation to study drug in Part 1, the most common grade 1-2 non-haematological (non-heme) adverse effects (AEs) are fatigue (n=40), diarrhea (n=25), rash (n=24), myalgia (n=22), oral mucositis (n=17), peripheral neuropathy (n=15), nausea (n=14), blurred vision (n=14), edema (n=13), constipation (n=12), headache (n=11), dry eyes (n=9), dizziness (n=9) and watery eyes (n=6). Grade 3 non-heme AEs included fatigue (n=3), nausea (n=0), rash (n=1), pleural effusion (n=1), infection (n=2) and dyspnea (n=1). There was no grade 4 or grade 5 non-heme toxicities in Part 1. In part 2, common grade 1-2 hematological (heme) AEs was anemia (n=13). Grade 3-4 haematological AEs included neutropenia (n=2), ALT increase (n=1) and febrile neutropenia (n=1). In Part 2, there was no grade 5 hematologic toxicity. The toxicity after intensive immune-chemotherapy in shortened cycles are much improved compared to historical controls but longer follow-up is needed.
Conclusions: Preliminary data indicate that the chemotherapy-free induction with ibrutinib and rituximab in newly diagnosed, young MCL patients was efficacious and well-tolerated. This unprecedented efficacy and safety may provide a window of opportunity for less chemo-immunotherapy needed for consolidation.
Preliminary findings form the Window Study: a phase II clinical trial
Preliminary findings form the Window Study: a phase II clinical trial
Wang:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Onyx: Research Funding; BeiGene: Research Funding; Pharmacyclics: Research Funding; Kite Pharma: Research Funding; Asana BioSciences: Research Funding; Juno Therapeutics: Research Funding; Acerta Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Fayad:Seattle Genetics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal