Abstract
Introduction: In the past several years significant progress has been made in the understanding of MM pathogenesis, drug resistance and the related role of the bone marrow microenvironment (BMM). Hence various treatment strategies are currently in (pre-) clinical use, targeting both malignant plasma cells and the BM niche. In February 2015 the first epigenetic modifying pan histone deacetylase inhibitor (HDACi), panobinostat, granted approval for relapsed or refractory MM. However there is insufficient data so far to conclusively unravel the complex interactions between "epi-drugs" and the rapid increasing number of antimyeloma agents. Thus, we assess the impact of novel drug combinations (NOX-A12, auranofin, class selective HDACis, Sirt-2 inhibitors) on the BMM in a suitable matrix-based 3D co-culture platform to best mimic in vivo conditions whilst ensuring easy handling.
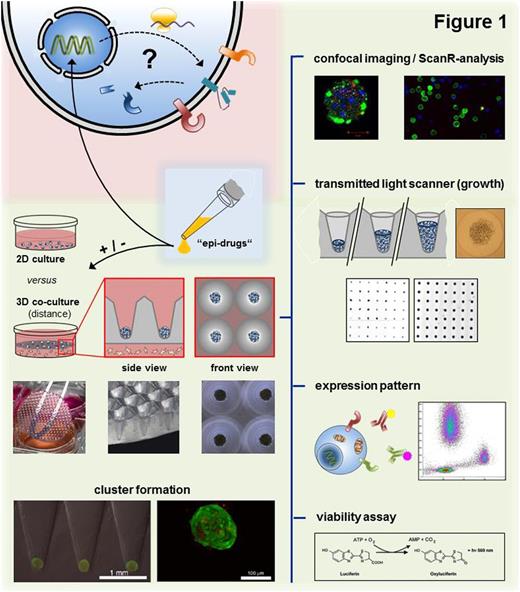
Methods: The innovative 3D co-culture platform is based on transparent agarose matrices with 2 mm deep conical micro-cavities, wherein semi-adherent cell populations can be either seeded in contact or distance co-culture (Fig. 1). Due to the uniquely defined conical shape of the cavities, proliferation as well as treatment response could be monitored up to several weeks by determining the circular area of the cell clusters via transmitted light scanner. To further compare common flat 2D versus 3D culturing with regard to treatment response and growing behavior, FACS analysis and viability assays were performed. In addition, we examined altered expression levels of adhesion molecules (CXCR4, CD138, CD38, Slam-F7) in terms of culture conditions and epigenetic modulations (class-selective HDACis) using multi-panel flow cytometry. Qualitative and quantitative distribution of fluorescence dyes (apoptosis, proliferation) inside the cell agglomerations was visualized using mCherry transduced RPMI-8226 cells combined with confocal imaging and ScanR-analysis.
Results: Human MM cell lines (MMCLs; U266 and RPMI-8226) exhibited substantial differences concerning growth and therapeutic responses under 2D versus agarose-based 3D culture conditions. MMCLs and especially primary MM patient samples could be cultured without reconditioning steps for more than three weeks under moderate logarithmic growth conditions inside the agarose matrices. Due to simple media and/or stromal exchange a loss or dying of cells could be consistently avoided. In contrast semi-adherent MMCLs (initial concentration 105 cells/ml) showed exponential proliferation in common flat culture until nutrient starvation induced apoptosis after approx. six days. Furthermore, malignant plasma cells became significantly resistant to proteasome inhibitors (PI) bortezomib (BTZ; 6nM, 48h) and auranofin (AUR; 3 µM, 48h) by forming solid 3D cell-agglomerations and almost remained unaffected if co-cultured additionally with HS-5 BM stromal cells (BMSCs). To exclude uneven distribution of agents, we determined the equal penetration of fluorescent dyes (CellTrace Violet/CFSE) into cell-clusters via confocal imaging.
Further data suggest a great impact of 3D co-culture conditions on the expression pattern of modern target structures per se, leading e.g. to a decrease in CXCR4 but persistence of CD138 levels if compared to 2D mono-culture. Moreover we demonstrated a significant decrease of CD138 in low toxic concentrations of panobinostat (8nM, 48h) indicating a intriguing modulation of the BMM by epigenetic modifying agents. For subsequent steps we already verified synergistic antimyeloma effects of the novel HDAC6-selctive inhibitor JS28 in combination with BTZ and aim to further elucidate the influences of various selective deacetylase inhibitors on the BM niche.
Conclusions and Ouline: Our recent data underline the importance of accurate 3D co-culture models to mimic in vivo proliferation and drug resistance and to predict later clinical treatment success more precisely. Based on this suitable platform, we are currently assessing the impact of cluster formation, stromal support and "epi-drugs" on the expression pattern of adhesion molecules to derive most rational drug combinations with modern target structures, such as CXCR4, CD138, CD38 and SlamF7 and to allow more effective targeting of the BMM.
Engelhardt:Janssen: Research Funding; MSD: Research Funding; Amgen: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal