Abstract
Background
Survival for acute promyelocytic leukemia (APL) has improved dramatically in recent decades through use of targeted therapy with all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). Early deaths, principally due to complications of APL coagulopathy, remain a significant barrier to cure. White blood cell (WBC) count at diagnosis is a well validated risk marker for early death and relapse. While APL is characterized by t(15;17), mutations of the FLT3 gene are prevalent. Prior reports in both adult and pediatric APL have demonstrated variable impact of FLT3 mutation status on outcomes. In a pediatric cohort from the Intergroup Study CALGB 9710, our group previously found an association of FLT3 mutations with early death. The more recent Children's Oncology Group (COG) APL trial AAML0631 included addition of two courses of arsenic trioxide (ATO) during consolidation. Relapse rate on this study was low, but survival, particularly for the high risk patients, was impacted by early deaths. In a prior analysis we reported that a significant proportion of patients on AAML0631 experienced clinically significant bleeding and clotting events. Here we report our analysis of the prevalence of FLT3 mutations and their association with outcomes for pediatric APL patients treated on the AAML0631 study.
Methods
Diagnostic bone marrow samples were collected from consenting patients with newly diagnosed APL enrolling onto COG AAML0631. FLT3 mutation testing of these bone marrow samples included evaluation for internal tandem duplications (FLT3/ITD) of the juxta-membrane domain and point mutations of the activating loop (FLT3/ALM) of the tyrosine kinase domain. FLT3 mutation status was correlated with clinically significant bleeding/clotting, early death during induction and relapse. Significance of correlation was determined using the chi-square test and relapse risk for patients who achieved CR by end of Induction 1 was calculated using cumulative incidence.
Eligible patients were age ≥ 2 years and < 22 years and required confirmation of PML-RARA by PCR testing. Risk group was defined by WBC at diagnosis: standard risk (SR) WBC < 10,000 and high risk (HR) WBC ≥ 10,000. Treatment regimen has been previously reported [Kutny et al, ASH Abstract, 2015].
Results
There were 101 evaluable patients on AAML0631 including 35 HR APL and 66 SR APL. A total of 94 patients had specimens available for FLT3 mutation testing. Patients with unknown FLT3 status (3 HR and 4 SR) were excluded from the following analyses. FLT3 mutations were present in 43% (N=40) including 35% (N=33) with FLT3/ITD, 4% (N=4) with FLT3/ALM, and 3% (N=3) with both FLT3/ITD and FLT3/ALM. The median WBC was 24,450 for FLT3 mutant APL compared to 7,130 for FLT3 wild type, and the rate of FLT3 mutation was significantly higher in HR APL compared to SR APL (66% vs. 31%, P=0.001). Four patients died during induction (all HR APL with WBC of 16200-173800), but early death did not correlate with FLT3 mutation (2/4 patients had FLT3 mutations). We analyzed association of FLT3 mutations with prevalence of clinically significant (Grade III-IV) bleeding or clotting events during induction. There was no difference in prevalence of coagulopathy events (N= 19) between those with and without FLT3 mutations (18% vs. 22%, P=0.57).
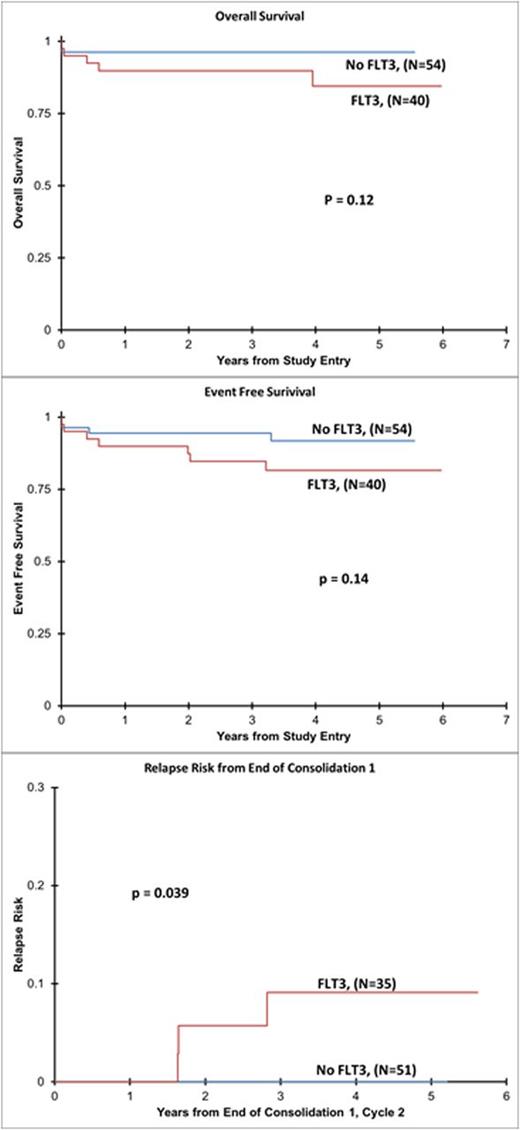
While OS and EFS were not significantly different, the relapse rate from end of Consolidation 1 was significantly higher at 6% (N=3) in FLT3 mutant patients versus 0% in FLT3 wild-type (P=0.039) [Figure 1].
Conclusions
FLT3 mutations are highly prevalent (43%) in pediatric APL. The COG AAML0631 study represents the second largest group of pediatric APL patients with outcome data treated on a cooperative group trial and to our knowledge the current report is the largest group of pediatric APL patients evaluated for outcome based on FLT3 mutation status. This study failed to show an association of FLT3 mutations with early death or bleeding/clotting events in induction. With ATO consolidation, the relapse rate was low on this trial. However, it is of great interest that the relapse rate following ATO consolidation was significantly higher in FLT3 mutant patients. Evaluation of a larger (international) cohort of pediatric APL patients is warranted to further define of role of FLT3 in relapse risk, but the current results give further support to consideration of integrating FLT3 inhibitors into relapsed/refractory APL treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal