Abstract
Patients with chemotherapy refractory non-Hodgkin lymphoma (NHL) have poor prognosis. We report the results of a phase 2 clinical trial testing the efficacy of haploidentical donor NK cell infusion with rituximab for refractory NHL. Sixteen patients were enrolled, and 14 were evaluable.
Treatment included IV lymphodepleting chemotherapy (pentostatin 3.75mg x 2 days; n=6 or fludarabine 25mg/m2 x 4 days; n=10) with cyclophosphamide (60mg/kg IV day -5; n=14). To enhance NK cell function and deplete regulatory T cells(Treg), we used the IL-2 receptor-targeting agent denileukin diftitox (18 μg/kg/day IV day -1; n=5) or methylprednisolone (1 mg/kg day -2 through day +9; n=9). Donor lymphocytes were CD3- and CD19-depleted and overnight stimulated with 1000 U/ml IL-2. The cellular product at a dose of 1.5-8 x 107 NK cells/kg was infused IV on day 0 followed by subcutaneous infusion of IL-2 at 9 million IU x 6 doses. Peripheral blood (PB) donor NK cells were evaluated by short tandem repeats and/or flow cytometry analyzing donor-specific HLA alleles.
Patients characteristics included median age of 52 years (range 48-67); diffuse large B cell lymphoma was the most common diagnosis (n=11); all patients were rituximab and chemotherapy refractory.
Of 14 evaluable patients at 2 months post-infusion, 4 achieved remissions (2 complete, 2 partial; 28%). Three patients in continuous remission proceeded to allogeneic donor transplantation at 2,3 and 9 months after NK cell therapy.
Compared to healthy controls, NK cells in patients prior to therapy had reduced function (CD107a, INFγ and TNFα production), lower expression of natural cytotoxic receptors NKp46, NKp44 and DNAM (but higher NKp30) and reduced expression of suppressor receptors TIGIT, PD1 and TIM3.
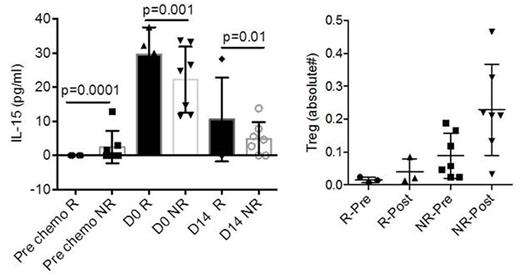
In all evaluable patients (n=9), donor NK cells persisted in the PB for at least 7 days after infusion with median 18% (range 0.5-97%) of the NK cells were of donor origin. In one responding patient, donor NK cells sustained beyond day 28. Responding patients (R) had significantly lower levels of absolute number of Treg (Foxp3+CD25+CD127-) at baselineand 14 days after therapy (Figure) as compared to non-responders (NR), however neither denileukin diftitox nor methylprednisolone resulted in PB Treg depletion. Expectedly, lower PB Tregs correlated with low serum levels of IL-10 (R2=0.7; p<0.001; n=12), suggestive of attenuated immuno-suppressive milieu. In addition, frequencies of PB myeloid derived suppressor cells (MDSC) were remarkably low in responders as compared to non-responders at baseline (4.5%±0.05% vs. 11%±6%) and after therapy (day 14: 3.8%±1%; vs. 9.4%±5%).
Endogenous Inteleukin-15 (IL-15) serum levels peaked at the day of NK cell infusion (range 11-33pg/dl). Responders had higher IL-15 levels compared to non-responders at day 0 (Figure). IL-15 levels also correlated with functional NK cell killing at day 14 as measured by expression of CD107a (R2=0.8; p=0.008; n=5), suggesting that higher endogenous IL-15 improved NK cell cytotoxic capacity.
We observed expected, but transient hematologic toxicity. Therapy was associated with grade 3 adverse effects (tumor lysis syndrome, Pneumocystis pneumonia; Clostridium difficile colitis, neutropenic fever; thrombotic microangiopathy). One unevaluable patient developed sepsis prior to NK cell infusion, and the second died of sepsis a week after therapy.
In summary, our observation supports further investigation of donor NK cellular therapies for advanced NHL as a strategy to overcome chemoresistance. NK cell expansion and therapeutic efficacy might be further improved by administration of IL-15.
Cooley:Fate Therapeutics: Research Funding. Miller:Oxis Biotech Scientific Advisory Board: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal