Abstract
Background: In both the novel and pre-novel agents era, high dose therapy followed by autologous hematopoietic cell transplant (AHCT) has been shown to prolong survival in multiple myeloma (MM) in randomized trials, but prospective have only included younger patients (65 -70 years and younger). Given that the median age at diagnosis of MM is 66 years, it is important to know the outcomes of AHCT in older patients. Similarly, the definite outcomes of AHCT in very young patients (<50 years) is also lacking as they represent a very small proportion in clinical trials.
Methods: We analyzed a consecutive cohort of patients with MM receiving ASCT from 2000-2015 in two different age groups, old (>70 years; N=105) and young (≤50 years, N=86) and compared the outcomes. The primary objectives were to assess overall survival (OS), progression free survival, (PFS), and non-relapse mortality (NRM) in these two groups.
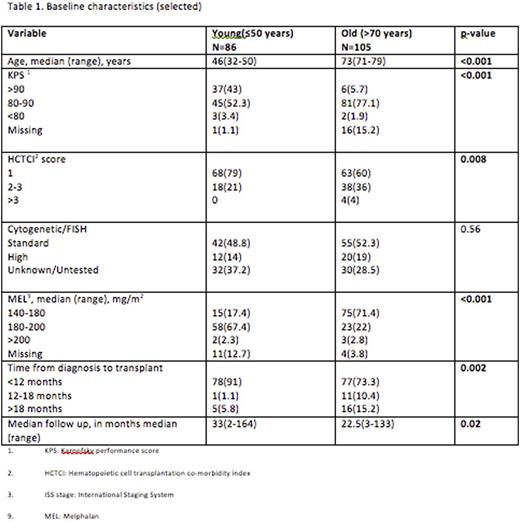
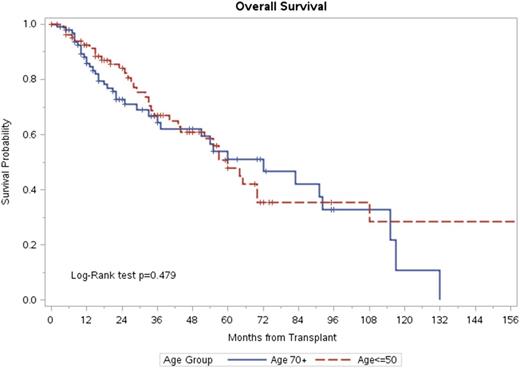
Results: Eighty-sex patients were young (≤50 years), while 105 patients were old (>70 years). Young patients had better performance status and lower co-morbidity index, while majority of older cohort received dose reduced high dose melphalan between 140-180 mg/m2. Median follow up of survivors in the younger cohort was 33 months (range, 2-164) compared to the 22.5 months (3-133) in the old group (p=0.02). The PFS at 1 year was 60% (95%CI 46-72) for young patients and 58 (95%CI, 45-69) for the old ones. The OS at 1 year was 92% (95% CI 84-96) for younger and 85% (95% CI 76-91) for the older cohorts. On multivariate analysis, age did not have any effect on survival (p =0.82), high-risk cytogenetics (HR 2.2, 95% CI 1.06-4.6, p=0.04) was associated with higher mortality. High-risk cytogenetics (HR 1.2, 95% CI 1.1-3.5, p=0.004) and non-responding or progressive disease at transplantation (HR, 5.0 95% CI 1.8-13.5, p=0.02) were significantly associated with inferior PFS.
Conclusion: Age by itself should not be a limiting factor in considering the modality of AHCT. However consideration could be given to augmenting therapy for young patients with additional novel treatments post transplant given their similar outcomes with the old patients based on our study.
Overall survival between the two age groups, 1: >70 years old and 2: ≤50 years old.
Overall survival between the two age groups, 1: >70 years old and 2: ≤50 years old.
Hamadani:Takeda: Research Funding. Hari:Merck: Research Funding; BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal