Abstract
Background: Despite major advances in the treatment of multiple myeloma (MM) only a minority of patients achieve long-term disease control. Immunotherapy combined with autologous hematopoietic stem cell transplantation (auto-HCT) may reduce relapse rates. Immunoglobulin idiotype (Id) conjugated with a carrier protein, Keyhole limpet hemocyanin (KLH), is a tumor-specific antigen in MM. Vaccine-primed, anti-CD3/anti-CD28 costimulated adoptive T-cell transfer can augment humoral and cellular immune responses to vaccination despite cytotoxic therapy. We hypothesized that Id-KLH vaccine + the vaccine-primed costimulated T cells will result in a robust Id-specific humoral and cellular response, compared to a control vaccine (KLH only).
Methods: In this randomized, phase II trial, the primary objective was to determine if Id-KLH primed, costimulated T cells will induce a more robust Id-specific immunity than KLH-primed T cells. Eligible patients had IgG monoclonal protein. Patients were randomized 1:1 to receive either Id-KLH vaccine or KLH-only vaccine, followed by auto-HCT, and then vaccine- primed costimulated T cells followed by two booster doses of the vaccine to which they were randomized. This study was supported by The MD Anderson Cancer Center SPORE in Multiple Myeloma.
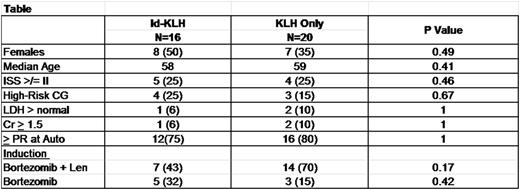
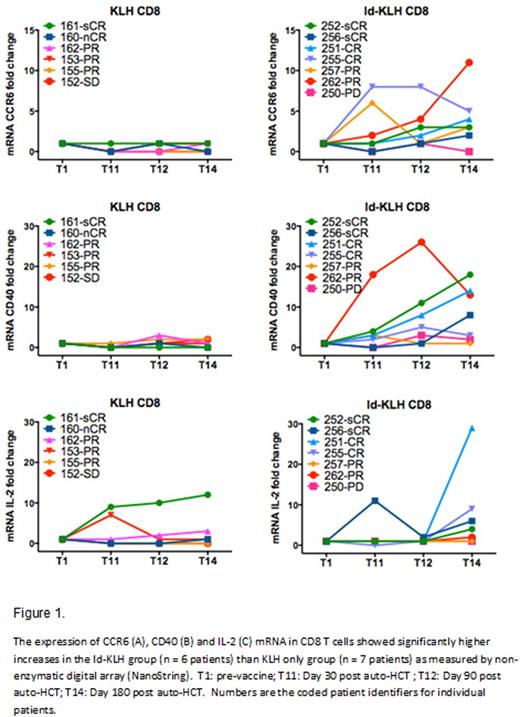
Results: A total of 36 patients were enrolled between 1/2013 and 5/2015. Sixteen (44%) were randomized to Id-KLH and 20 (55%) to KLH-only. The Table below summarizes the patient characteristics. There was no significant difference between the two groups in terms of age, risk status or induction therapy. No treatment-related mortality, infusion reactions or dose-limiting toxicity was seen in either arm. Five (31%) and 3 (15%) patients achieved complete remission (CR) by day+180 in the Id-KLH and KLH arms, respectively (p=0.42). Initial analysis of a subgroup of patients revealed a significantly higher mRNA expression of immune activation genes IL-2, CCR6 and CD40 by NanoString nCounter in the Id-KLH group compared with the KLH only group (Figure). Eleven (68%) and 17 (85%) went on to receive maintenance therapy with lenalidomide or lenalidomide + ixazomib in the Id-KLH and KLH arms, respectively (p=0.42). After a median follow up of 26 months, 2-year PFS was 81% and 83% in Id-KLH and KLH arms, respectively (p=0.35).
Conclusion: Id-KLH vaccine and vaccine-primed costimulated T cells can be safely administered in the setting of auto-HCT.There was a more robust immune response and a trend towards higher CR rate in the Id-KLH group.
Stadtmauer:Teva: Consultancy; Amgen: Consultancy; Novartis: Consultancy; Takada: Consultancy; Janssen: Consultancy; Celgene: Consultancy. Garfall:Medimmune: Consultancy; Bioinvent: Research Funding; Novartis: Consultancy, Research Funding. Cohen:Janssen: Consultancy; Bristol-Meyers Squibb: Consultancy, Research Funding. June:Johnson & Johnson: Research Funding; Immune Design: Consultancy, Equity Ownership; Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; University of Pennsylvania: Patents & Royalties; Celldex: Consultancy, Equity Ownership; Tmunity: Equity Ownership, Other: Founder, stockholder ; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal