Abstract
Introduction: The number of available salvage agents for patients with relapsed ALL has significantly increased in the last 5 years. Furthermore, these recent therapies, such as antibody and T-cell therapy, have a distinct mechanism of action and toxicity profile compared to conventional multi-drug chemotherapy combinations which have been the mainstay of ALL treatment. We sought to investigate the potential efficacy and toxicity of these agents when used prior to allogeneic hematopoietic cell transplantation (HCT), and to compare overall transplant outcomes with these different types of therapies.
Methods: 126 consecutive patients with ALL in second complete remission (CR2) underwent HCT at MD Anderson Cancer Center between January 2004 and December 2015. The patient and transplant characteristics are described in Table 1. The probabilities of outcomes were calculated with the Kaplan-Meyer method. Variables found to be significant at the p<0.1 level were included in a Cox regression multivariate model. All p values are two sided.
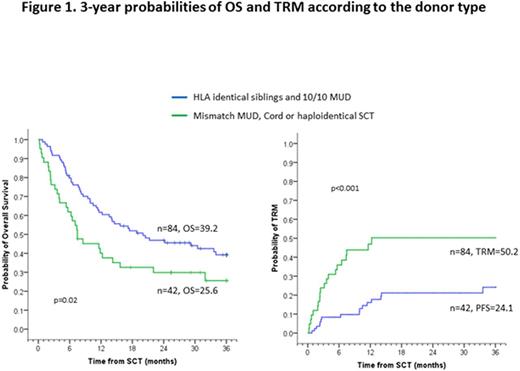
Results: With a median follow-up of 38.4 months (range 6-125), the 3-year overall survival(OS), progression-free survival (PFS) and transplant-related mortality (TRM) rates were 34.6%, 29.5% and 33.2%, respectively, for the entire group. Patients receiving well-matched transplant donors fared better than those receiving mismatched donors (Figure 1). Second CR was attained in 104 patients with the first line of salvage therapy. Salvage therapy consisted of HyperCVAD-based chemotherapy alone in 57 patients, chemotherapy plus tyrosine kinase inhibitors (TKI) in 18, chemotherapy plus rituximab in 11, chemotherapy plus inotuzumab in 5, inotuzumab monotherapy in 12, and blinatumomab monotherapy in one patient. We found no statistical difference in OS, PFS or TRM following transplant based on these prior therapies. Furthermore, we found no difference in the expected probabilities of acute or chronic GVHD. In the 8 patients treated with blinatumomab monotherapy for first or subsequent salvage, all 8 progressed following transplant at a median of 4.5 months.
Eight out of 126 (6.3%) patients developed VOD; 3 out of 26 (11.5%) patients treated with inotuzumab developed VOD, but this did not impact TRM post HCT compared with other treatment groups.
Conclusions: Transplant outcomes were not different following antibody or T-cell salvage therapy as compared to conventional chemotherapy in this single center, retrospective study. Larger numbers of patients will need to be studied as we continue to incorporate these newer therapies into our treatment regimens.
Ciurea:Cyto-Sen Therapeutics: Equity Ownership; Spectrum Pharmaceuticals: Other: Advisory Board. Jabbour:BMS: Consultancy; ARIAD: Consultancy; Pfizer: Research Funding; Pfizer: Consultancy; Novartis: Research Funding; ARIAD: Research Funding. Kantarjian:ARIAD: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Champlin:Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal