Abstract
Introduction: Patients (pts) with Chronic Lymphocytic Leukemia (CLL) are known to have an increased risk of other cancers. Treatment with lenalidomide has been associated with an increased rate of solid tumors (ST) in pts with multiple myeloma (MM). However, the occurrence of ST in patients with CLL receiving therapy with lenalidomide remains unknown.
Methods: We evaluated the development of ST in pts with CLL that received front-line treatment with lenalidomide as monotherapy or in combination with rituximab in clinical trials conducted at our institution. We report the characteristics and timing of ST, as well as patient outcome. All pts enrolled had indication for therapy according to the International Workshop on CLL guidelines and no history of malignancy for three years, with the exception of treated malignancy with indolent behavior such as prostate cancer that was treated with surgery or radiation therapy.
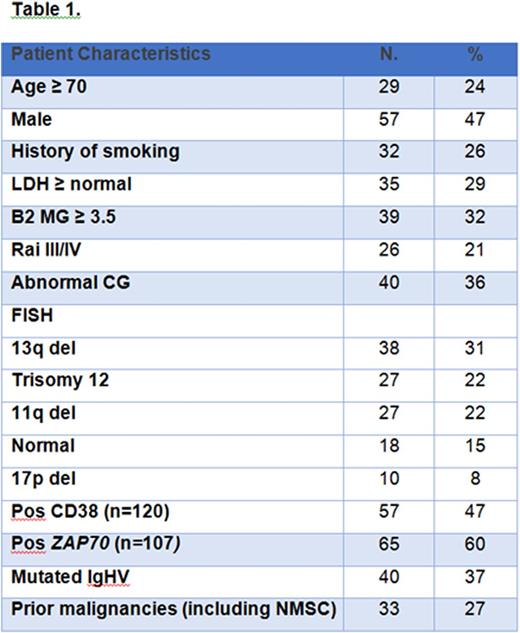
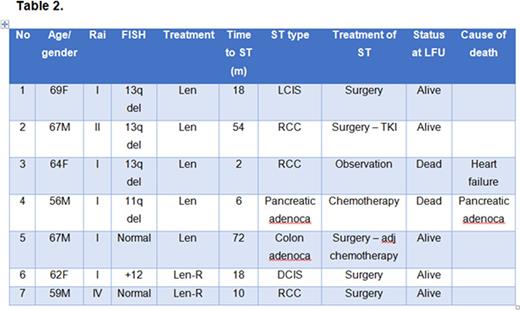
Results: One-hundred twenty-one pts were enrolled in two consecutive phase II clinical trials of frontline therapy with lenalidomide (N = 61, 51%) or combination of lenalidomide and rituximab (N = 60, 49%). Baseline pts characteristics are shown in Table 1, median age was 66 yrs and 24% of patients were age 70 or older. At a median follow up after lenalidomide therapy of 41 months (range 1-102+), 7 (6%) pts developed a ST: renal cell carcinoma (RCC, 3 pts), localized breast cancer (LCIS and DCIS, 2 pts), pancreatic adenocarcinoma (1 pt) and colon adenocarcinoma (1 pt) (details are shown in Table 2). The median time interval between receiving lenalidomide-based therapy and the development of ST was 1.5 years (range 0-8.5). At the time of this report, 5 out of 7 pts with ST are alive and cancer-free. Two pts died: 12 months and 18 months after developing ST from progression of pancreatic adenocarcinoma and heart failure, respectively.
Since pts with CLL have a high rate of non-melanoma skin neoplasms (NMSC), we also monitored patients for new diagnoses of skin cancers. Seven (6%) additional pts developed NMSC, 4 of these pts had prior history of NMSC. All cases of NMSC were superficial and did not require systemic therapy.
Additionally, because of the high median age of this population, we reviewed prior history of malignancies. Eleven (9%) pts had history of ST [prostate cancer (N = 9), bladder cancer (N = 1), RCC (N = 1)]. Twenty-two pts (18%) had skin cancers (NMSC, (N = 21) and 1 pt had history of melanoma. None of them had received chemotherapy, two had radiation therapy [prostate cancer = 1, NMSC (basal cell carcinoma of the nose (1 pt)]. All ST were in remission before starting therapy with lenalidomide for more than 3 years as per the inclusion criteria.
Conclusions: Seven cases of ST were observed in our population of 121 pts with CLL that received initial therapy with lenalidomide, after a median follow-up of 3.5 years. The most common neoplasm was RCC followed by in-situ breast cancer. In our limited experience the timing, number and type of malignancies do not appear to mirror what was seen in patients with MM. However, this comparison may be limited by the size of our patient population and length of follow-up. Our group reported the incidence of ST in patients treated with FCR. After a similar follow-up the incidence of second malignancies was similar (5.9%), but the type of malignancies was different, although NMSC remained the most common ST in both experiences.
Reporting the incidence of other malignancies in patients with CLL enrolled in large studies, including those of novel targeted agents, will help understanding the expected incidence of ST and how novel treatments modulate such risk.
Jain:Pharmacyclics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding; Infinity: Research Funding; Abbvie: Research Funding; Genentech: Research Funding; Incyte: Research Funding; Servier: Consultancy, Honoraria; Novimmune: Consultancy, Honoraria; BMS: Research Funding; Novartis: Consultancy, Honoraria. Thompson:Pharmacyclics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal