Abstract
Acute leukemia (AL) of ambiguous lineage (AMBI-L) comprises up to 5% of AL cases in both children and adults. Although several definitions exist, a general treatment guideline has been missing. Single country studies usually report fewer than 50 cases of children or adults. Accordingly, the international iBFM AMBI2012 Study/Registry collected 275 AMBI-L cases in patients <18y from Australia, Austria, Brazil, Czechia, Germany, Greece, Israel, Italy, Netherlands, NOPHO (Denmark, Estonia, Finland, Norway, Sweden, Iceland, Latvia and Lithuania), PINDA (Chile), Poland, SAHOP (Argentina), Slovakia, St. Jude's Children Research Hospital (USA), Texas Children's Cancer Center (USA), Ukraine and United Kingdom. Each center/country reported all consecutive patients with AMBI-L from a 2 to 13 year period ending May 31, 2015. Apart from the study itself, the central database served also as a basis for consulting individual patients during the diagnostic workup. Preliminary results of this study were first introduced in ASH 2015 and now the complete detailed analysis of updated findings including significance of immunophenotype, molecular genetics, blast clearance and transplant are shown.
In total, 275 patients were included in the study. Of these, 240 fulfilled the definitions of biphenotypic/mixed phenotype AL, partially overlapping with cases in whom two clones had been identified (n=68) and 15 cases presented with undifferentiated AL. Most patients started their treatment with an ALL-type protocol (n=161), 79 with AML therapy, 27 with a combined regimen, including the Interfant protocols, 2 patients were not treated, 2 received other treatment, and in 4 patients such information was missing. The 5yEFS of the entire cohort was 56±3.7% and 5y overall survival was 67±3.3%. Patients treated by ALL-type protocols had superior 5 year event free survival (5yEFS) (70±4.6%, n=158) compared to those who started AML-type treatment (5yEFS: 40±6.4%, n=78) or hybrid ALL/AML treatment (5yEFS: 50±11%, n=27). Although protocol selection was likely biased, we recommend ALL treatment, when diagnostic findings, including molecular genetics, fail to indicate AML therapy.
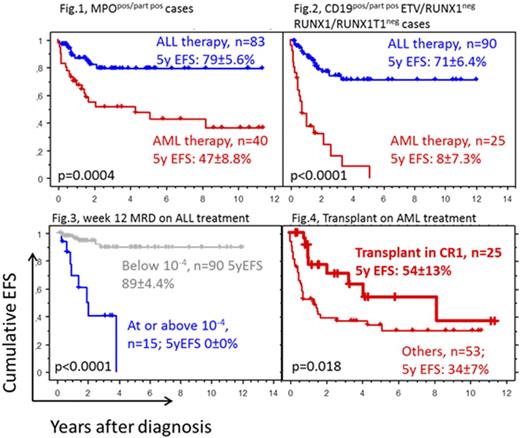
Although myeloperoxidase (MPO) has been used as the ultimate marker of myeloid lineage, patients who started with ALL-type treatment demonstrated a better prognosis even among cases classified as MPOpos/part pos (Fig. 1). These differences by initial choice of treatment are most prominent when CD19pos/part pos cases are analyzed regardless of the overall lineage (Fig. 2). This shows that at least for CD19pos/part pos cases in the absence of RUNX1/RUNX1T1 fusion, treatment should not start with current AML-type protocols.
Until week 12, patients with higher leukemia burden were slightly overrepresented compared to non-AMBI ALL patients (data not shown). In addition, patients with higher residual disease had a much poorer prognosis. Thus, Prednisone poor and good responders (based on day 8 blood blast counts) had a 5yEFS of 50±9.7%, n=38 and 81±5.8%, n=82, respectively (p=0.005). By day 15 bone marrow (BM), only cutoffs of 10-4 and 10-3 were analyzed and neither showed significant associations with EFS. At the end of induction, patients with BM residual disease ≥10-3 had a 5yEFS of 51±10%, n=49 compared to 90±4.3% for those with lower levels, n=75 (p=0.0002). Especially higher residual disease at week 12 was associated with an extremely poor EFS (Fig. 3). Early identification of patients with inadequate response and designing alternative treatment for them is our important challenge.
No overall benefit of transplantation was seen in patients who started on ALL treatment or hybrid ALL/AML treatment. Again, this may be caused by a biased selection of more severe cases for transplant. In patients who started with AML treatment, transplant appeared to improve prognosis (Fig. 4).
This study provides the basis for improved treatment of future patients with AMBI-L, with more accurate diagnostics.
OH, AL, IJ, EM and JS were supported by Czech Health Research Council 15-28525A.
Bleckmann:JazzPharma: Other: financial support of travel costs. Moricke:JazzPharma: Honoraria, Other: financial support of travel costs. Inaba:Arog: Research Funding. Kattamis:Novartis: Honoraria, Research Funding; ApoPharma: Honoraria. Reinhardt:Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Jazz Pharma: Other: Travel Accomodation; Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal