Abstract
Introduction: CD4+CD25+FoxP3+ regulatory T cells (Treg) have broad suppressive activity and play a central role in the maintenance of immune tolerance and prevention of chronic graft-versus-host disease (cGVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Interleukin-2 (IL-2) is a key growth factor for Treg and we previously reported that daily administration of low-dose IL-2 in patients with refractory cGVHD induces selective expansion of Treg and NK cells. No expansion of CD4 conventional T cells (Tcon) or CD8 T cells was noted. In a Phase 2 study, 64% of patients had clinical responses. Predictors of clinical response included younger age, early IL-2 initiation and a higher Treg:Tcon ratio at study baseline and at week 1 of IL-2 therapy. However, there were no significant differences in overall Treg expansion or plasma IL-2 levels between responders and non-responders during the initial 12 week treatment period. We therefore asked whether functional or gene expression differences in Treg or effector T cells could distinguish clinical response to IL-2.
Methods: In vitro Treg suppression assays were performed using cryopreserved patient samples obtained at study baseline and week 6 of IL-2 therapy. CD4+CD25+CD127- Treg were cultured with CellTrace Violet-labeled CD4+CD25- Tcon in the presence of stimulating antibody-coated beads. Proliferation was measured as CellTrace Violet dilution by flow cytometry after 4 days of incubation. All patient samples were tested against the same healthy donor (HD) Treg and Tcon. Differential gene expression between clinical responders and non-responders was examined by RNA Seq analysis of sorted Treg, Tcon, CD8 and NK cells at study baseline and week 4 of IL-2 therapy.
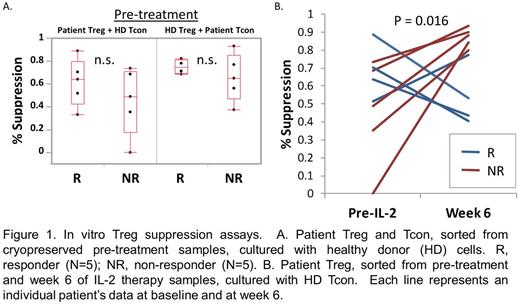
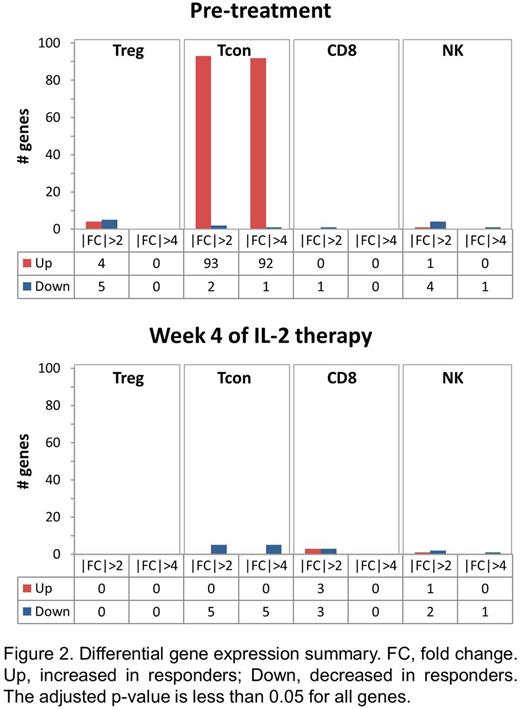
Results: To compare Treg cell function between response groups, we tested the ability of patient Treg to suppress the proliferation of HD Tcon. To determine whether there were qualitative differences in effector T cells between the response groups, we measured suppression of patient Tcon by HD Treg. At study baseline, clinical responders appear to have higher Treg suppressive function although the differences between the response groups were not significant due to small sample size (Figure 1A). Patient Tcon in responders and non-responders were similarly suppressed by HD Treg. At week 6 of IL-2 therapy, Treg from non-responders showed significant improvement in suppressive activity against HD Tcon, whereas there was no significant change in suppressive function of Treg from responders (Figure 1B). Thus, despite the lack of clinical improvement in cGVHD symptoms, non-responder Treg show both numeric increase and functional improvement in response to IL-2 therapy. To identify other cell-intrinsic differences that distinguish clinical response to IL-2, we performed RNA Seq on sorted Treg, Tcon, CD8 and NK cells in 3 responders and 3 non-responders. Differentially expressed genes were identified using the DEseq package. There were very few differentially expressed genes between response groups within the pre-treatment Treg, CD8 and NK cell samples (Figure 2). In contrast, 93 genes (92 upregulated, 1 downregulated) with greater than 4-fold difference in expression levels were identified in pre-treatment Tcon samples between responders and non-responders. The top 10 gene ontogeny terms associated with these differentially expressed genes include cell-cell adhesion and ion transport processes. By week 4 of IL-2 therapy, the gene expression profiles of responder and non-responder cells were very similar. There were no differentially expressed genes within Treg, and only 5 within Tcon.
Conclusions: In addition to increasing absolute Treg numbers, daily low-dose IL-2 improves Treg suppressive function in patients who do not exhibit clinical cGVHD improvement. Interestingly, RNA Seq results suggest that the determinants of clinical response do not lie within the Treg. Instead, the greatest differences between response groups were detected in pre-treatment CD4 Tcon, implying that lack of clinical response reflects resistance of CD4 effector cells to suppression mediated by Treg in vivo. Further analysis of differentially expressed Tcon genes may help elucidate the mechanisms of resistance to IL-2 therapy in patients with cGVHD.
Koreth:LLS: Research Funding; prometheus labs inc: Research Funding; kadmon corp: Membership on an entity's Board of Directors or advisory committees; amgen inc: Consultancy; millennium pharmaceuticals: Research Funding; takeda pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Armand:Otsuka: Research Funding; Roche: Research Funding; BMS: Consultancy, Research Funding; Infinity: Consultancy; Merck & Co., Inc.: Consultancy, Research Funding; Sequenta: Research Funding; Sigma Tau: Research Funding; Tensha: Research Funding. Soiffer:Kiadis: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Ritz:Kiadis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal