Abstract
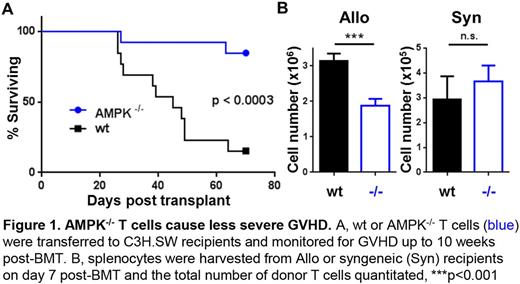
Allogeneic hematopoietic stem cell transplantation (alloHSCT) represents a curative treatment for high-risk leukemia and a number of non-malignant hematologic disorders. However, the therapeutic use of alloHSCT remains limited by acute graft-versus-host disease (GVHD), where activated donor T cells attack and destroy host tissues in the skin, gastrointestinal tract, and liver. We have previously shown that the alloreactive T cells responsible for GVHD increase their dependence on the oxidation of fat relative to either syngeneic or naive T cells. To explore this adaptation mechanistically, we studied the role of AMPK, an intracellular energy sensor and known driver of fat oxidation, in donor T cells during GVHD. Alloreactive T cells increased phosphorylation of AMPK as early as day 3 post-transplant, with up-regulation in pathways both up- and downstream of AMPK. Changes in phosphorylation were up to 8-fold higher in alloreactive T cells compared to naive T cells or syngeneic controls (p=0.0003). We then investigated the role of AMPK during GVHD pathogenesis using donor cells deficient in AMPK (from AMPKα1fl/flα2fl/fl x CD4-Cre mice). AMPK-/- T cells caused significantly less GVHD in both major-MHC and minor-histocompatibility mismatch models of GVHD (Figure 1A), with a coordinated decrease in the number of donor T cells recovered on day 7 post-transplant (3.15 +/- 0.49x106 versus1.87 +/- 0.53x106, p=0.0006, wildtype (wt) versus AMPK-/- respectively). Importantly, expansion of syngeneic T cells was unaffected by AMPK deficiency (Figure 1B).
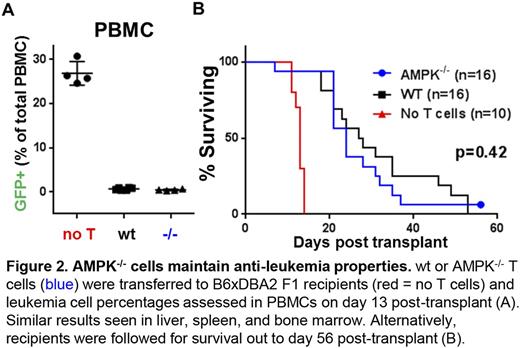
We next investigated the ability of AMPK-/- T cells to mount effective cytotoxic and anti-leukemia responses. AMPK-/- T cells demonstrated equivalent cytotoxicity against MHC-mismatched targets both in vitro and in vivo and differentiated in similar proportions into cytokine-producing cells (IFN-γ, TNFα, IL-17, and IL-4). We then assessed graft-versus-leukemia (GVL) potential in AMPK-/- cells using a GVL model with high tumor burden. AMPK-/- T cells exhibited equivalent clearance of p815 leukemia cells on day 13 post-transplant (Figure 2A), and extended survival of recipient mice similarly to wt T cells (Figure 2B). To elucidate possible mechanisms underlying this separation of GVL and GVHD responses, we evaluated metabolic pathways in wt and AMPK-/- T cells recovered on day 7. To our surprise, rates of fatty acid oxidation were identical between wt and AMPK-/- T cells and loss of AMPK did not impact alloreactive T autophagy, nor impair signaling downstream of mammalian target of rapamycin.
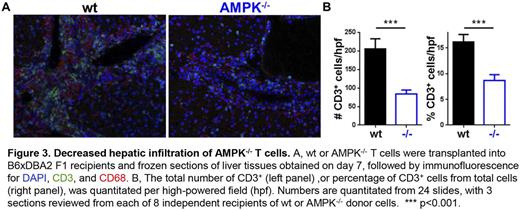
To define the mechanism underlying AMPK-/- benefits, we quantitated levels of regulatory T cells (Treg) on day 7 post-transplant. In contrast to expectation, both the percentage and total number of Treg increased in mice receiving AMPK-/- T cells (0.85 +/- 0.32x104 vs. 1.69 +/- 0.34x104, wt vs. AMPK-/-, p=0.004). Loss of AMPK facilitated donor Treg expansion, as elimination of FoxP3+ cells prior to transplantation abrogated differences between wt and AMPK-/- donors on day 7. Importantly, Treg levels were equal in wt versus AMPK-/- donors prior to transplantation. Finally, we assessed the ability of AMPK-/- T cells to infiltrate into GVHD target organs. As shown in Figure 3, peri-portal infiltration of AMPK-/- cells was significantly reduced compared to wt T cells, and infiltrates in recipients of AMPK-/- cells contained many fewer CD3+ T cells per high-powered field, with CD3+ cells representing a lower percentage of cells overall. Decreased hepatic infiltration correlated with a lower percentage of circulating CD4+ cells and lower levels of the integrin pair α4β7 (55.2 +/- 1.4% versus 47.2 +/- 2.8% α4β7Hi cells, p=0.0017, wt vs. AMPK-/-).
In conclusion, deletion of AMPK in donor T cells decreases GVHD severity but spares anti-leukemia responses and preserves homeostatic immune reconstitution. Mechanistically, this occurs through a decrease in pathogenic T cell numbers, an increase in the number and percentage of Treg cells, fewer circulating CD4+ cells, and decreased infiltration of donor cells into target organs. From these findings, we conclude that AMPK represents a clinically relevant target in donor T cells pre-transplant and are actively exploring ways to translate this exciting therapy into clinical practice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal