In this issue of Blood, Yang et al investigate the mechanism behind the aging-related increase in venous thrombosis and provide evidence for the novel role of mammalian target of rapamycin complex 1 (mTORC1) in mediating the observed increase in mean platelet volume, a predictor of unprovoked venous thromboembolism.1-3
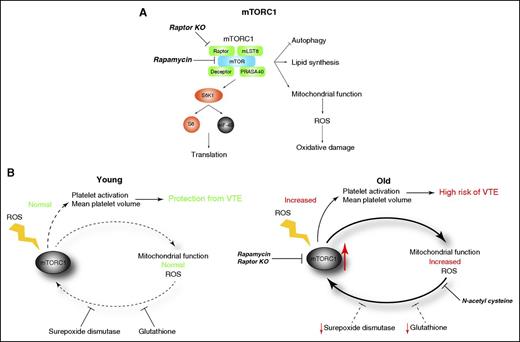
Diagrammatic representation of downstream signaling driven by the mTORC1. (A) Targeting mTOR with rapamycin or knockout of raptor prevents activation of mTORC1, and therefore prevents increased mitochondrial function and reactive oxygen species production. (B) Model of mTORC1 activation. In the young, cellular redox is maintained by adequate production of the antioxidants superoxide dismutase and glutathione, and competent mitochondrial function prevents venous thromboembolism (left). During aging, the levels of superoxide dismutase and glutathione decrease, and mitochondrial function deteriorates, resulting in the accumulation of reactive oxygen species. Hyperactivation of mTORC1 by reactive oxygen species results in further oxidative stress and a vicious cycle of oxidant production. Targeting mTORC1 function or antioxidant therapy contributes to reduced risk of venous thrombosis. KO, knockout; ROS, reactive oxygen species.
Diagrammatic representation of downstream signaling driven by the mTORC1. (A) Targeting mTOR with rapamycin or knockout of raptor prevents activation of mTORC1, and therefore prevents increased mitochondrial function and reactive oxygen species production. (B) Model of mTORC1 activation. In the young, cellular redox is maintained by adequate production of the antioxidants superoxide dismutase and glutathione, and competent mitochondrial function prevents venous thromboembolism (left). During aging, the levels of superoxide dismutase and glutathione decrease, and mitochondrial function deteriorates, resulting in the accumulation of reactive oxygen species. Hyperactivation of mTORC1 by reactive oxygen species results in further oxidative stress and a vicious cycle of oxidant production. Targeting mTORC1 function or antioxidant therapy contributes to reduced risk of venous thrombosis. KO, knockout; ROS, reactive oxygen species.
As the world population ages, the incidence of age-related venous thromboembolism is increasing. In the United States, the incidence is 300 000 to 600 000 cases a year, with a 10% to 30% mortality rate, and costing up to $10 billion per year.4 Venous thromboembolism is now the third leading cause of death in the United States; therefore, further characterization of the mechanisms of venous thromboembolism is of critical public health concern.
mTORC1 is a multiprotein complex consisting of mTOR, raptor, mLST8, deceptor, and PRASA40 (see figure, panel A), and is essential for platelet production.5 Recent studies have shown that mTORC1 is a key regulator of aging.6 During aging, mean platelet volume increases,7 which is associated with hyperactivity. In addition, mTORC1 activation is associated with the detrimental effects of aging in the cardiovascular system, including the heart, blood vessels, and hematopoietic stem cells.8 Because mean platelet volume is a predictor of mortality in venous thromboembolism and heart diseases, and mTORC1 activity increases with age, Yang et al hypothesized that mTORC1 dysregulation during aging plays a role in alteration of platelet production leading to the observed age-dependent increase in mean platelet volume and increased susceptibility to venous thromboembolism.
To determine if mTORC1 is involved in venous thromboembolism, Yang et al assessed the effect of rapamycin, an inhibitor of mTORC1, on megakaryocyte (MK) size, mean platelet volume, and experimental deep vein thrombosis in mice. Old (16-month-old) mice have an increase in MK size, mean platelet volume, and experimental deep vein thrombus size, compared with young (4-month-old) mice. Administration of rapamycin for 2 months decreased MK size, mean platelet volume, and experimental deep vein thrombosis in the old mice, to levels approaching those of the young mice. Rapamycin treatment had no effect on these parameters in the young mice. Because of the potential off-target effect of whole-body dosing of rapamycin, the authors assessed the effect of mTORC1 knockout. For this, they used a platelet-specific knockout of raptor that prevents the formation of mTORC1. Similar to the rapamycin results, old raptor knockout mice showed decreased MK size, mean platelet volume, and experimental deep vein thrombosis compared with their wild-type littermates of the same age. These data demonstrate the novel role of mTORC1 in promoting age-related increase in MK size, mean platelet volume, and venous thromboembolism.
Aging is a complex process that is not yet fully understood. However, one driving factor is thought to be the increase in unbalanced redox state. During aging, levels of the antioxidants superoxide dismutase and glutathione decrease, whereas mitochondria accumulate oxidative damage due to an increase in reactive oxygen species. mTORC1 can be activated by reactive oxygen species and this mTORC1 hyperactivation has been proposed to drive reactive oxygen species accumulation.9 This scenario results in a vicious cycle of mTORC1 activation followed by increased reactive oxygen species production (see figure, panel B).
If indeed, reactive oxygen species accumulation drives mTORC1 activation (and vice versa), then suppressing the accumulation of reactive oxygen species would curb mTORC1 activation. To test this, the authors assessed whether antioxidant therapy could prevent the age-related thrombotic phenotype. Treatment of aging mice with the antioxidant N-acetyl-l-cysteine prevented the age-dependent increase in MK size, mean platelet volume, and venous thromboembolism, demonstrating that reducing reactive oxygen species production is beneficial. Conversely, treatment of young mice with dl-buthionine-(S,R)-sulfoximine, which inhibits the production of the antioxidant glutathione and increases reactive oxygen species, resulted in a phenotype similar to aged mice.
In summary, Yang et al provide evidence that aging results in accumulation of reactive oxygen species and hyperactivation of mTORC1 that precipitates a vicious cycle of upregulated mTORC1 signaling that, in the context of platelet function, correlates with increased risk of venous thromboembolism. These novel insights for the first time offer a mechanism behind the regulation of mean platelet volume, and may lead to the adoption of antioxidants such as N-acetyl-l-cysteine, resveratrol, or mitochondrial-targeted antioxidants as a prophylactic or adjunct therapy for venous thromboembolic diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.