Abstract
The introduction of the Bruton tyrosine kinase (BTK) inhibitor ibrutinib has dramatically changed the management of chronic lymphocytic leukemia (CLL). Although responses have been durable in the majority of patients, relapses do occur, especially in the high-risk patient population. Most relapses occur as the result of acquired mutations in BTK and PLCG2, which may facilitate success with alternative targeted therapies. As outcomes after ibrutinib relapse have been reported to be poor, specific strategies are needed for this patient population. Here, I discuss the diagnosis and management of ibrutinib-refractory CLL. The focus will be on common clinical scenarios that can be mistaken for relapse and how to accurately determine which patients are relapsing. Because there is no established standard of care, I discuss currently available options for standard therapy and existing clinical data. I also discuss new agents with the potential to be effective in patients refractory to ibrutinib. Finally, I discuss strategies for long-term disease control in this patient population.
Introduction
The management of chronic lymphocytic leukemia (CLL) has changed dramatically during the last 5 years, with the development and subsequent US Food and Drug Administration approval of the Bruton tyrosine kinase (BTK) inhibitor ibrutinib. Before the introduction of kinase inhibitors in CLL, patients with relapsed disease had relatively poor progression-free survival (PFS) and overall survival (OS) with standard therapies.1-5 In the phase 1b/2 study of ibrutinib in patients with relapsed or refractory CLL, at a median follow-up of 30 months, PFS was 69% and OS 79%.6 The phase 3 trial that led to the approval of ibrutinib in relapsed CLL compared it with the CD20 monoclonal antibody ofatumumab. With a median follow-up of 9.4 months, both PFS (median, 8.1 months for ofatumumab vs not reached for ibrutinib) and OS (12-month estimates: 81% for ofatumumab vs 90% for ibrutinib) were significantly improved with ibrutinib.7
For patients with treatment-naive CLL, the outcomes with ibrutinib have been even more promising. The longest follow-up in the front-line setting is for 31 patients aged 65 years or older treated as part of the phase 1b/2 PCYC 1102 study.6 With a median follow-up of 35.2 months, median PFS was not yet reached, and 30-month PFS was 96%. The 1 patient who progressed developed Richter’s transformation at 8 months. In the phase 3 Resonate II trial, ibrutinib was compared with single-agent chlorambucil; for ibrutinib, 18-month PFS was 90%, and 24-month OS was 98%.8
Multiple studies have also reported outcomes for high-risk patients treated with ibrutinib. A phase 2 study has been performed in patients with TP53 aberrations with either treatment-naive or relapsed disease. The cumulative incidence of progression at 24 months was 9% for previously untreated and 20% for previously treated patients. Estimated OS at 24 months was 84% for previously untreated and 74% for previously treated patients.9 Similarly, the phase 2 Resonate 17 study investigated patients with relapsed/refractory CLL and del(17p). In this group, 12-month PFS was 79.3%, and OS was 84%.10
Although these remarkable data make it tempting to think that patients might be able to be treated with ibrutinib for the rest of their natural lives, patients do indeed relapse, and ibrutinib-refractory CLL is becoming an increasingly prevalent clinical problem. Here I address some of the intricacies of detecting and treating ibrutinib-refractory CLL.
Defining ibrutinib resistance
Detecting progression in patients receiving continuous ibrutinib therapy is not always straightforward. Because most patients will not attain a complete response (CR), and many will have circulating leukemia cells for long periods of time, determining which patients are indeed relapsing can be a challenge. Following are some commonly encountered clinical scenarios.
Case 1
Patient is a 53-year-old man who started ibrutinib 7 months ago for treatment of relapsed CLL. His response thus far with ibrutinib is a partial response with lymphocytosis (PR-L), and absolute lymphocyte count (ALC) has been steadily decreasing, with a last recorded value of 20 000/μL 1 month prior. He was feeling well until about 4 days ago, when he began to have a low-grade fever, myalgias, cough, and new cervical adenopathy. He has no sick contacts. On exam, he has bilateral cervical nodes 2 × 2 cm and no other adenopathy. White blood cell count is 36, with an ALC of 30.
This is a common scenario, and this patient most likely has an upper respiratory infection leading to cervical adenopathy and increased white blood cells. A rise in the lymphocyte count is seen frequently with infection, especially in patients with residual lymphocytosis on ibrutinib. The response category PR-L, into which this patient falls, is a newer designation resulting from kinase inhibitor therapies that result in lymphocyte mobilization, where patients may meet criteria for a partial response by node resolution and blood count improvement long before lymphocytosis resolves.11 PR-L has not been shown to be an inferior response in terms of remission duration.12 In this case, ibrutinib should be continued. Because constitutional symptoms can occasionally herald the development of Richter’s transformation in these patients, I would have the patient return if symptoms persist longer than a week, and would consider rechecking complete blood count in 1 month.
Case 2
Patient is a 75-year-old man with relapsed CLL who has been taking ibrutinib for 2 years. He has attained a partial response, with residual small abdominal nodes and low-level bone marrow involvement with CLL, but normal peripheral counts. He is planning to have a knee replacement and discontinues ibrutinib 7 days before the procedure. On the morning of the procedure, he notes 3 days of night sweats and fatigue, white blood cell count of 2, hemoglobin of 9.4, and platelets of 110. He is concerned that these symptoms are similar to those he experienced before initiating ibrutinib and wonders whether the drug is not working. He notes that he held ibrutinib 3 months ago for a dental procedure with no symptoms.
Clearly, in this case the patient has developed signs of disease progression while holding the drug, so he is not resistant to ibrutinib. The drug should be reinstated as soon as possible, and response can be expected quickly. Symptomatic disease progression on ibrutinib withdrawal appears to be most common earlier in therapy and in patients with higher levels of residual disease, but cannot always be predicted. For these patients, appropriate dose interruptions for procedures should be performed, but drug discontinuations need to occur for as short of time as possible. Steroids can occasionally be used for palliation until ibrutinib is restarted.
Case 3
Patient is a 50-year-old woman with CLL receiving ibrutinib as her third-line therapy. She has been receiving treatment for 3 years and has been feeling well. On routine exam, you note that she has no palpable lymphadenopathy, but ALC has increased from 3000/μL to 6000/μL. She reports compliance with ibrutinib. You ask her to return in 2 months, and at that time, ALC has further risen to 8000/μL. She remains asymptomatic, but you note new 1.5-cm lymph nodes in the neck.
This patient is relapsing on ibrutinib with an increasing white blood cell count and the presence of new palpable adenopathy. It is important to closely monitor patients who show any signs of disease progression so that relapse can be caught early. Although with other therapies this would prompt an immediate discontinuation of therapy, it has been shown that the tempo of relapse tends to escalate when ibrutinib is discontinued,13 so ibrutinib should be continued until the next therapy is started. If a wash-out period is necessary before a next-line clinical trial, the patient will need to be watched closely for disease escalation during the period off ibrutinib.
These cases serve to highlight the challenge faced in defining disease progression in patients receiving continuous ibrutinib therapy, especially those patients who have dose interruptions for toxicities or procedures. It is essential in these patients to show progression on repeated evaluations so as to not abandon an effective therapy. As well, it is also imperative that ibrutinib not be discontinued in a relapsing patient until a new plan is in place.
Epidemiology and natural history of ibrutinib resistance in CLL
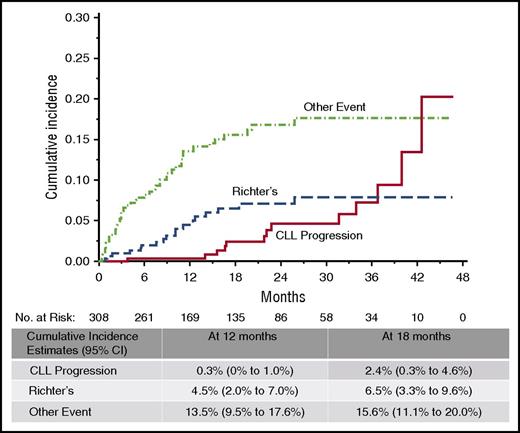
Relapse on ibrutinib occurs in 2 forms: progressive CLL and histologic transformation, most commonly to large cell lymphoma or prolymphocytic leukemia. Transformation generally occurs within the first 2 years of therapy, whereas CLL progression almost never occurs during the first year of treatment, and the incidence continues to increase with time (Figure 1). Baseline karyotypic complexity on stimulated karyotype appears to be the most significant independent predictor for ibrutinib relapse with CLL13,14 ; however, as data mature from the larger clinical studies, it is likely that other risk factors will also emerge. Factors that have been shown to be important with other therapies, such as number of prior treatments and del(17p), have been risk factors for progression in univariable analysis, but not in multivariable analysis,13 but the importance of these may increase with longer follow-up.
Cumulative incidence of ibrutinib discontinuation. Rate of discontinuation is low overall, with relapse-related discontinuations less frequent than nonrelapse during the first 3 years of therapy. Richter’s transformation tends to occur earlier than progression with typical CLL. CI, confidence interval. Reproduced from Maddocks et al13 with permission.
Cumulative incidence of ibrutinib discontinuation. Rate of discontinuation is low overall, with relapse-related discontinuations less frequent than nonrelapse during the first 3 years of therapy. Richter’s transformation tends to occur earlier than progression with typical CLL. CI, confidence interval. Reproduced from Maddocks et al13 with permission.
CLL relapse on ibrutinib is primarily mediated through the acquisition of mutations in BTK or its immediate downstream target, PLCG2.13,15-17 BTK C481S, the most common acquired mutation in BTK, reduces the binding affinity of ibrutinib for BTK and changes ibrutinib from an irreversible to a reversible inhibitor.15,18 The mutations identified in PLCG2 have all been demonstrated to be potentially gain-of-function, allowing activation through the BCR even in the presence of inactive BTK.15,19 Mutations in BTK and/or PLCG2 are present in 85% to 90% of patients at relapse when using high-sensitivity assays.17,20 Clonal evolution has also been shown to be a hallmark of ibrutinib resistance, and other groups have noted a recurrent deletion in 8p in patients at the time of relapse.21
Current data suggest that patients with acquired resistance to ibrutinib have a poor survival, although it is likely that many of the earlier patients did not have the opportunity to receive newly emerging highly effective therapies. In our institutional cohort of patients, median survival after CLL relapse was 23 months.17 Other sites have reported even poorer outcomes of 5.7,22 3.1,23 and 3 months24 after ibrutinib discontinuation. These outcomes clearly show that ibrutinib-refractory patients are an especially high-risk population, and to manage these patients effectively, we must think beyond our standard CLL therapies.
It is important to differentiate ibrutinib resistance from ibrutinib intolerance, as patients who discontinue ibrutinib therapy because of adverse effects have a very different natural history.17 There are no data to suggest these patients will not do well on alternate therapy, and they do seem to have the potential to respond to either idelalisib25 or venetoclax.26
Clinical data for treatment of patients after ibrutinib relapse
A number of novel agents have been studied in patients previously treated with ibrutinib (Table 1). The most promising data thus far are with the BCL2 inhibitor venetoclax. In a study of single-agent venetoclax for patients previously treated with kinase inhibitors, ORR among patients refractory to ibrutinib was 70%.26 Unlike the non–ibrutinib refractory population, the CR rate appears to be relatively low, with a reported CR of only 2%. Forty-five percent of patients achieved minimal residual disease negativity in the peripheral blood, and 1 patient achieved minimal residual disease–negative bone marrow. Presented data show an estimated 12-month PFS of 80% for patients previously treated with either ibrutinib or idelalisib.26 Previous data with venetoclax suggest remission duration is associated with depth of remission, so this may mean remission durations will not be as long as for those patients who do not have ibrutinib-refractory disease; however, this remains to be seen, as the data so far are very encouraging.
Agents clinically evaluated in patients refractory to ibrutinib
| Agent . | Number of ibrutinib-refractory patients . | Median duration of treatment . | ORR, % . |
|---|---|---|---|
| Venetoclax | 43 | 13 mo | 70 |
| Duvelisib | 6 | 4.1 cycles | 16.6 |
| Entospletinib | 15 | 16 wk | 28* |
| Agent . | Number of ibrutinib-refractory patients . | Median duration of treatment . | ORR, % . |
|---|---|---|---|
| Venetoclax | 43 | 13 mo | 70 |
| Duvelisib | 6 | 4.1 cycles | 16.6 |
| Entospletinib | 15 | 16 wk | 28* |
ORR, overall response rate.
Refers to all prior BTK inhibitor-treated patients, not just those with ibrutinib-refractory disease.
Upregulation of PI3K is commonly seen in ibrutinib-resistant lymphoma,27 suggesting that idelalisib-based therapy may be successful in the ibrutinib-refractory population. Preclinical data with the PI3K p110 γ/δ inhibitor duvelisib also suggests this agent may be effective in patients with C481S BTK mutations.28 Preliminary results with duvelisib have not shown striking efficacy29 ; however, patients were not necessarily stratified according to BTK mutational status. A “real-world” experience of patients treated with idelalisib on ibrutinib resistance showed an ORR of 28% with a median PFS of 8 months.25
The Syk inhibitor entospletinib (GS-9973) is currently under investigation in a cohort of patients previously treated with BTK inhibitors (predominantly ibrutinib, but 1 patient with spebrutinib).30 Preclinical rationale exists for this approach, with data suggesting that inhibition of Syk can overcome PLCG2 mutations,31 and thus likely could also be beneficial in the presence of upstream mutations. Preliminary data showed a response rate of 28% among patients previously treated with a BTK inhibitor, with a median exposure of 16 weeks (range, 1-60 weeks) for patients who had ibrutinib-resistant disease.
Other promising drugs and targets
Because the mutations that have been associated with relapse do not significantly alter the BCR pathway, alternative targeting of the BCR pathway at or downstream of sites of mutation may be an effective strategy to treat relapsed patients. In patients with acquired mutations in BTK, alternative targeting of BTK with a reversible inhibitor that binds outside of C481 may be an ideal therapy. GDC-0853,32 ARQ-531,33 and SNS-06234 are all reversible BTK inhibitors that have shown preclinical efficacy; however, no clinical trial data are yet available. For those with PCLG2 mutations or BTK mutations, other downstream targets such as PKCβ might also be clinically relevant. Preclinical testing with sotrastaurin has shown proof of concept in this area35 ; however, clinical studies are not being pursued in this population with this particular agent.
Also of particular interest are agents that target BTK in a manner distinct from ibrutinib. Among potential others, this includes HSP90 inhibitors, HDAC inhibitors, and XPO1 inhibitors. BTK is a client protein of HSP90, and HSP90 inhibitors have been shown to overcome ibrutinib resistance in vitro.36 HDAC inhibitors have been shown to inhibit BTK protein expression through upregulation of BTK-targeting microRNAs and show cytotoxicity and signaling inhibition in cells with C481S BTK.37 Similarly, the XPO1/CRM1 inhibitor selinexor has been shown to suppress BTK gene expression and to be effective in cell lines with BTK C481S mutations, as well as an in vivo model of ibrutinib resistance.38
Certainly there are other agents and pathways with the potential for utility in this group of patients, and further study is needed to identify promising targets.
My approach to treatment of patients who relapse while receiving ibrutinib or those at risk for relapse
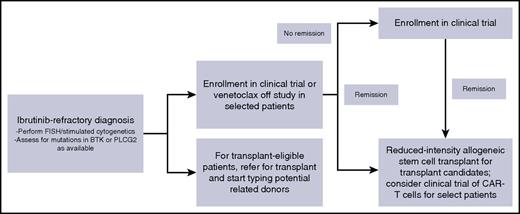
When I see patients in clinic who are relapsing while receiving ibrutinib, we discuss short-term disease management and long-term disease control (Figure 2). To control disease in the short term, I favor enrollment on a clinical trial whenever possible. Because the absolute number of patients with ibrutinib-resistant disease is small, trials specifically for this patient population are especially appealing to gain specific knowledge in this population and to move the field forward. I always repeat fluorescence in situ hybridization and cytogenetics at the time of relapse. For patients with del(17p), venetoclax is a very reasonable option for standard therapy, and for patients without del(17p), idelalisib plus rituximab is a reasonable consideration. Given the limited PFS reported with PI3K inhibitors in this setting, I would view idelalisib best used as a bridge to a long-term strategy. Data with venetoclax in this setting appear more promising and, for patients who achieve a complete response, may be a definitive therapy. I do not consider chemoimmunotherapy or single-agent CD20 antibodies to be a reasonable intervention for these patients; although no formal data exist, anecdotal reports have not indicated efficacy. For long-term disease control, I discuss reduced-intensity stem cell transplant for all those who are eligible by age, performance status, and organ function. For those who are ineligible for or not interested in transplant, I would consider a clinical trial of CAR-T cells as a reasonable long-term strategy.
My approach to ibrutinib-refractory CLL. CAR-T, chimeric antigen receptor T; FISH, fluorescence in situ hybridization.
My approach to ibrutinib-refractory CLL. CAR-T, chimeric antigen receptor T; FISH, fluorescence in situ hybridization.
Patients who relapse with Richter’s transformation represent a particular challenge, as there are no specific data in this population, and outcomes are dismal. For the few patients without complex karyotype or who have clonally unrelated Richter’s, I will use chemoimmunotherapy, usually R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) based on data showing particularly good outcomes in this patient population,39 although other chemoimmunotherapy regiments can be considered as well. For all other patients, I will choose a clinical trial of a novel agent if available. Checkpoint inhibitors have been especially interesting in this patient population.40 All eligible patients with Richter’s transformation after ibrutinib should be considered for transplant.
For patients with baseline complex karyotype in whom I am considering therapy with ibrutinib, I generally offer a clinical study of a BTK inhibitor–based combination study in addition to standard-of-care ibrutinib. Even though this group is still likely to have a >1-year remission duration on ibrutinib, I think it is important to discuss the risk for relapse on ibrutinib up front, and many patients are willing to accept the potential added toxicity of a combination therapy to potentially improve the remission duration on ibrutinib. As well, for young fit patients with multiply relapsed disease and complex karyotype, it is reasonable to consider stem cell transplantation during remission achieved with ibrutinib. However, as options for next-line therapy are expanding, this option should be reserved for select high-risk patients.
Finally, at our center, we have begun to screen all patients every 3 months for BTK and PLCG2 mutations and have found that detection of these mutations reliably predicts patients that will go on to relapse. In the context of a clinical trial, we will offer the addition of a novel agent to ibrutinib to patients who develop an ibrutinib-resistance mutation in an attempt to prolong remission duration. Whether this strategy does indeed prolong remission duration remains to be seen; however, if effective, it would certainly be preferable to prevent relapse than to treat uncontrolled CLL.
Conclusions
At the current time, ibrutinib resistance is an uncommon but growing problem. As more research is performed to determine which patients are at risk for relapse on ibrutinib, it will be important to identify high-risk patients a priori and consider these patients for combination therapies or use ibrutinib as a bridge to a stem cell transplant or other long-term intervention. As well, the identification of biomarkers of impending relapse may allow for salvage therapies or combination strategies before the accelerated disease progression associated with relapse. Although the introduction of BTK inhibitors has represented a massive leap forward in the therapy of CLL, it is clear that there is still work to be done to improve the survival and quality of life for our patients with CLL.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute grants K23 CA178183, R01 CA197870, and R01 CA183444. J.A.W. has received research support from Acerta, Karyopharm, and Morphosys.
Authorship
Contribution: J.A.W. wrote this paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Jennifer A. Woyach, The Ohio State University, 445A Wiseman Hall CCC, 410 W 12th Ave, Columbus, OH 43210; e-mail: jennifer.woyach@osumc.edu.