Key Points
The combination of ATRA and ATO, with or without GO, is effective and safe for newly diagnosed APL patients, including the high-risk subset.
Long-term follow-up suggests the responses are durable, with very rare relapses.
Abstract
The combination of all-trans-retinoic acid (ATRA) plus arsenic trioxide (ATO) has been shown to be superior to ATRA plus chemotherapy in the treatment of standard-risk patients with newly diagnosed acute promyelocytic leukemia (APL). A recent study demonstrated the efficacy of this regimen with added gemtuzumab ozogamicin (GO) in high-risk patients. We examined the long-term outcome of patients with newly diagnosed APL treated at our institution on 3 consecutive prospective clinical trials, using the combination of ATRA and ATO, with or without GO. For induction, all patients received ATRA (45 mg/m2 daily) and ATO (0.15 mg/kg daily) with a dose of GO (9 mg/m2 on day 1) added to high-risk patients (white blood cell count, >10 × 109/L), as well as low-risk patients who experienced leukocytosis during induction. Once in complete remission, patients received 4 cycles of ATRA plus ATO consolidation. One hundred eighty-seven patients, including 54 with high-risk and 133 with low-risk disease, have been treated. The complete remission rate was 96% (52 of 54 in high-risk and 127 of 133 in low-risk patients). Induction mortality was 4%, with only 7 relapses. Among low-risk patients, 60 patients (45%) required either GO or idarubicin for leukocytosis. Median duration of follow-up was 47.6 months. The 5-year event-free, disease-free, and overall survival rates are 85%, 96%, and 88%, respectively. Late hematological relapses beyond 1 year occurred in 3 patients. Fourteen deaths occurred beyond 1 year; 12 were related to other causes. This study confirms the durability of responses with this regimen.
Introduction
During the last 2 decades, all-trans-retinoic acid (ATRA) has revolutionized the treatment of patients with acute promyelocytic leukemia (APL), decreasing the 5-year mortality rate from 82% to 36%.1 Combination of ATRA with chemotherapy has been the standard of care in the treatment of APL, offering a complete remission (CR) rate of up to 90% to 95%, with cure rates exceeding 80%.2-7 Despite high CR rates, the induction mortality and relapse rates remain high, at 10% and 20% to 30%, respectively, underscoring the need to further improve patient outcomes.6,8-10
Arsenic trioxide (ATO) has a potent antileukemic effect in APL because of its effective targeting of PML-RARA-positive leukemic stem cells.11 Several studies have confirmed the efficacy of ATO in the treatment of recurrent APL after prior ATRA-based regimens.12-15 ATO, as a single agent, has also induced durable remissions in patients with newly diagnosed APL and has improved outcomes when added as consolidation therapy.16-18 Given the synergism between ATRA and ATO and the inherent potential morbidity and mortality of intensive chemotherapy, the combination of ATRA and ATO has been evaluated as an alternative to ATRA plus chemotherapy and has been of particular interest in older patients and those less able to tolerate cytotoxic chemotherapy.19-21
Several clinical trials have evaluated ATRA- and ATO-based induction regimens and have shown that the combination is safe and effective and superior to ATRA plus chemotherapy, at least in low- to intermediate-risk patients.10,22-26 However, inferior CR and survival rates have been reported for high-risk patients, defined by white blood cell (WBC) counts higher than 10 × 109/L, and for low-risk patients who develop leukocytosis (>10 × 109/L) during treatment with ATRA and/or ATO, with differentiation syndrome being a major concern for the use of such chemotherapy-free approaches.16,24,25,27,28
Gemtuzumab ozogamicin (GO) is an anti-CD33 monoclonal antibody conjugated to the toxin, calicheamicin, with significant activity in APL because of the high-density cell surface membrane expression of the CD33 antigen on APL cells.29 GO has been shown to be effective when added to ATRA in newly diagnosed patients with APL (CR rate, 84%) and as a single agent in patients with molecularly relapsed APL.30-33
Our group has conducted 3 consecutive trials combining ATRA plus ATO with the addition of GO in patients with high-risk disease and those low-risk patients who develop leukocytosis during the initial therapy. We have previously reported that the combination of ATRA plus ATO, with or without GO, is an effective regimen with an overall response rate of 92%, including a CR rate of 81% in high-risk patients.25 These results have been recently confirmed by a multicenter, phase 3 study conducted by the UK National Cancer Research Institute AML working group.34
Long-term follow-up studies demonstrating the durability of this “chemotherapy-free” regimen are limited. Here, we report the long term outcome of 187 patients newly diagnosed with APL treated in our institution, including 54 patients with high-risk disease.
Materials and methods
Patient population
Newly diagnosed patients with APL treated at our institution on 3 consecutive prospective clinical trials using the combination of ATRA and ATO, with or without GO (ID01-014; NCT01409161; NCT00413166), were reviewed. The studies were conducted according to the standards of good clinical practice, per institutional research policies and procedures and in accordance with the Declaration of Helsinki. Approval for these studies was obtained from the University of Texas MD Anderson Cancer Center’s institutional review board.
Eligible patients had to be 10 years or older and had newly diagnosed APL based on the presence of t(15;17) by standard cytogenetic analysis or by the presence of the PML-RARA fusion gene by reverse-transcriptase polymerase chain reaction (RT-PCR). Exclusion criteria were pregnancy, pretreatment QTc interval (corrected using Fridericia’s formula) greater than 480 ms unrelated to electrolyte imbalance; hepatic and renal dysfunction (as evidenced by serum creatinine >2.5, total bilirubin ≥2, and aspartate aminotransferase and alanine aminotransferase >3 × upper limit of normal) unless related to APL, hemolysis, or Gilbert’s disease. All patients provided a written informed consent.
Study design and treatment regimen
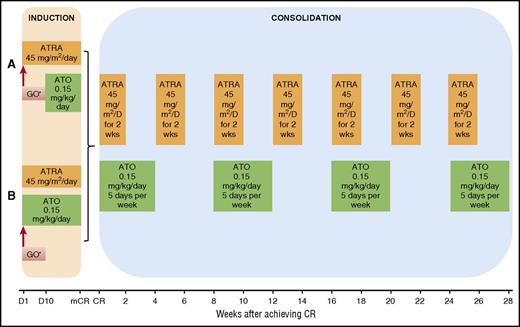
Details of the regimen have been previously published.25 Low-risk patients (WBC count ≤10 × 109/L) were initially treated with ATRA 45 mg/m2 in 2 divided doses daily and, beginning 10 days later, ATO 0.15 mg/kg intravenously daily (regimen A; Figure 1A). The induction regimen was modified in subsequent studies so that all patients received concomitant therapy with ATRA and ATO on day 1 (regimen B; Figure 1B). High-risk patients (defined by WBC count > 10 × 109/L on presentation) were treated identically except for additionally receiving 1 dose of GO 9 mg/m2 or idarubicin (IDA) 12 mg/m2 on day 1 (allowed because of a brief period of lack of availability of GO). Low-risk patients in whom the WBC count increased to more than 10 × 109/L during the first 4 weeks of therapy also received a dose of GO 9 mg/m2 or IDA 12 mg/m2 (allowed because of a brief period of lack of availability of GO). A bone marrow examination to assess response was performed between days 21 and 28 and repeated weekly, if necessary. Treatment was continued until there were less than 5% blasts and no abnormal promyelocytes in the bone marrow, after which treatment was discontinued until the achievement of CR with normalization of peripheral blood counts. Once in CR, patients received consolidation, as previously published (Figure 1).25 Disease monitoring and management of minimal residual disease were performed as previously reported.25
Treatment regimen. (A) Details of induction course, regimen A. (B) Details of induction course, regimen B. *One dose of GO 9 mg/m2 was given on day 1 for high-risk patients (defined by WBC count >10 × 109/L on presentation) and low-risk patients in whom the WBC count increased to more than 10 × 109/L during the first 4 weeks of therapy. D, day; mCR, marrow complete remission; wks, weeks.
Treatment regimen. (A) Details of induction course, regimen A. (B) Details of induction course, regimen B. *One dose of GO 9 mg/m2 was given on day 1 for high-risk patients (defined by WBC count >10 × 109/L on presentation) and low-risk patients in whom the WBC count increased to more than 10 × 109/L during the first 4 weeks of therapy. D, day; mCR, marrow complete remission; wks, weeks.
Supportive care
All patients received standard supportive care in the form of prophylactic and therapeutic antibiotics and transfusion of blood products to maintain a platelet count higher than 30 × 109/L, serum fibrinogen higher than 150 mg/dL, and internationalized normal ratio for prothrombin time less than 1.5. Methylprednisolone 50 mg intravenously daily for 5 days, followed by rapid taper starting on day 6, was administered to all patients to prevent APL differentiation syndrome.
Definitions and study end points
Patients were risk stratified according to the initial presenting WBC count, using a modified classification into a low-risk group (WBC count, ≤10 × 109/L) and a high-risk group (WBC count, >10 × 109/L).27,28 CR was defined by the presence of less than 5% blasts and no abnormal promyelocytes in the bone marrow in addition to platelet count 100 × 109/L or higher and neutrophil count 1.0 × 109/L or higher in the peripheral blood. Relapse events included hematological relapse, defined by either the presence of more than 5% blasts plus abnormal promyelocytes in bone marrow or evidence of extramedullary disease, and confirmed molecular relapse: PML-RARA detected by RT-PCR in 2 consecutive bone marrow samples performed 2 to 4 weeks apart. The study end points included overall survival (OS), event-free survival (EFS), and disease-free survival (DFS). OS was calculated from the start of therapy to death or last follow-up. EFS was calculated from the start of treatment to the date of relapse, death, or last follow-up, and DFS was measured from the date of CR until relapse or death from APL, with patients censored at the time of death in CR or last follow-up.
Safety assessment
Adverse events were continuously assessed, from the start of therapy to 30 days after discontinuation or completion of study treatment, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events versions 3.0 and 4.0. In addition, any serious adverse event that occurred after the 30-day period that was related to the study treatment was also reported. There was a 50% reduction in the dose of ATRA in patients who developed drug-related grade 3 to 4 toxicity (eg, headaches or rash), with drug discontinuation if toxicity persisted despite dose reduction. Also, ATO was reduced to 0.10 mg/kg in patients in CR who experienced a decrease in platelets to fewer than 75 × 109/L or neutrophils to fewer than 0.75 × 109/L unrelated to disease relapse, and in patients who developed atrial or simple ventricular arrhythmia unrelated to electrolyte imbalance. However, patients were removed from the study if the arrhythmia recurred or was ventricular tachycardia unrelated to electrolyte imbalance.
Statistical analysis
The survival curves were plotted using Kaplan-Meier method and compared using the log-rank test. After covariate adjustment, χ-squared and Mann-Whitney U tests were used to analyze differences in the distribution of variables among patient subgroups.
RT-PCR analysis
Initially, PML-RARA transcripts were analyzed using qualitative RT-PCR. However, after April 2005, quantitative RT-PCR was implemented. Details of the processing of bone marrow and blood samples for RNA extraction and RT-PCR protocols for PML-RARA amplification have been previously reported.25
Results
Patient characteristics
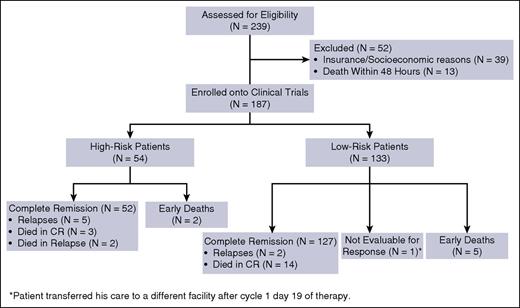
From July 2002 to May 2015, a total of 239 patients with newly diagnosed APL presented to our institution; 187 patients were enrolled into the 3 clinical trials. Reasons for excluding the remaining 52 patients (22%) were insurance/socioeconomic issues in 39 patients (75%) and death within 48 hours of presentation in 13 patients (25%) (Figure 2). Fifty-four patients (29%) had high-risk disease and 133 patients (71%) had low-risk disease, as defined earlier. Baseline and demographic characteristics of the patients are summarized in Table 1. The median age was 50 years (range, 14-84 years), with 52 patients (28%) at or above the age of 60 years. Median leukocyte count at presentation was 2.2 × 109/L (range, 0.3-187.9 × 109/L). Eighty-nine percent of patients (N = 167) had t(15:17) detected with standard cytogenetic analysis, of which 45 patients (24%) had additional cytogenetic abnormalities. Of note, the most common additional cytogenetic abnormalities were trisomy 8, detected in 11 patients (6.6%); inversion 9 in 4 patients (2.4%); and complex karyotype in 14 patients (8%). The translocation was absent in 20 patients (11%) who had a diploid or hyperdiploid karyotype or insufficient metaphases, or did not have the test performed. PML-RARA was detected by RT-PCR in all the patients, with the long isoform present in 105 patients (56%), short isoform in 78 patients (42%), and both isoforms in 4 patients (2%). Mutational gene analysis was performed in a variable number of patients with the following detected: FLT3-ITD mutation was positive in 59 of 152 patients (39%; high risk: 66%, low risk: 28%), FLT3-D835 mutation was positive in 18 of 153 patients (12%; high risk: 13%, low risk: 11%), RAS mutations were detected in 7 of 145 patients (5%; high risk: 2%, low risk: 6%), and CEBPA mutation was detected in 1 of 39 patients (3%). Other mutations including NPM1, C-KIT, JAK2, IDH1, and IDH2 were all negative in the tested patients. The median follow-up in surviving patients was 47.6 months (range, 2.7-159.7 months).
Patient characteristics at diagnosis
| Characteristics (N = 187) . | Number (%) . | Median (range) . |
|---|---|---|
| Age, y | 50 (14-84) | |
| ≥60 | 52 (28) | |
| Sex | ||
| Male | 97 (52) | |
| Female | 90 (48) | |
| Risk category | ||
| High-risk | 54 (29) | |
| Low-risk | 133 (71) | |
| Leukocyte count (×109/L) | 2.2 (0.3-187.9) | |
| Platelet count (×109/L) | 36 (3-261) | |
| Cytogenetics | ||
| t(15;17) | 122 (65) | |
| t(15;17) + other cytogenetic abnormalities | 45 (24) | |
| Diploid (RT-PCR +) | 10 (5) | |
| Not done/insufficient metaphases (RT-PCR+) | 9 (5) | |
| Hyperdiploid (RT-PCR +) | 1 (0.5) | |
| FAB morphology | ||
| M3 | 163 (87) | |
| M3v | 22 (12) | |
| Unknown | 2 (1) | |
| PML-RARA isoforms | ||
| Short | 78 (42) | |
| Long | 105 (56) | |
| Both | 4 (2) |
| Characteristics (N = 187) . | Number (%) . | Median (range) . |
|---|---|---|
| Age, y | 50 (14-84) | |
| ≥60 | 52 (28) | |
| Sex | ||
| Male | 97 (52) | |
| Female | 90 (48) | |
| Risk category | ||
| High-risk | 54 (29) | |
| Low-risk | 133 (71) | |
| Leukocyte count (×109/L) | 2.2 (0.3-187.9) | |
| Platelet count (×109/L) | 36 (3-261) | |
| Cytogenetics | ||
| t(15;17) | 122 (65) | |
| t(15;17) + other cytogenetic abnormalities | 45 (24) | |
| Diploid (RT-PCR +) | 10 (5) | |
| Not done/insufficient metaphases (RT-PCR+) | 9 (5) | |
| Hyperdiploid (RT-PCR +) | 1 (0.5) | |
| FAB morphology | ||
| M3 | 163 (87) | |
| M3v | 22 (12) | |
| Unknown | 2 (1) | |
| PML-RARA isoforms | ||
| Short | 78 (42) | |
| Long | 105 (56) | |
| Both | 4 (2) |
FAB, French-American-British.
Efficacy and response rates
Overall, 179 patients achieved CR after the induction course, for an overall CR rate of 96%. The CR rates for both low-risk and high-risk patients were equal at 96%. A confirmed molecular remission was achieved in 176 patients (98%); the remaining 3 patients were not assessed for molecular remission because of withdrawal of consent (N = 2) and loss to follow-up (N = 1). The median time to achieve CR was 30 days (range, 17 to 80 days), and the median time to molecular CR was 119 days (range, 20 to 277 days). Fifty-three patients with high-risk disease received cytoreductive therapy with induction; 45 patients (83%) received GO and 7 patients (13%) received IDA (12 mg/m2 1 dose only) during the period of lack of availability of GO. One patient (2%) received both GO and IDA (12 mg/m2 daily for 3 days) because of persistent leukocytosis despite receiving GO. One patient with a presenting WBC count of 11.1 × 109/L did not receive cytoreductive therapy despite having high-risk disease. Among the low-risk group, 96 patients (72%) developed leukocytosis with induction, with the median peak WBC of 19.8 × 109/L (range, 10.3-195) reached at a median of 10 days (range, 2-26 days) from the start of treatment. Of these, 60 patients received cytoreductive therapy in the form of GO (N = 51) and IDA (N = 9) at a median of 8 days (range, 2-14 days) from start of therapy with a rapid decline in the WBC count in all the patients. The remaining 36 patients did not receive cytoreductive therapy despite elevation in WBC count. Among the low-risk patients who developed leukocytosis, FLT-3 ITD mutation was detected in 19 of 77 tested patients (25%). No patients required the addition of GO for molecularly persistent disease.
Seven patients (4%) relapsed (5 of whom were high risk and 2 low risk), including 3 patients (2%) who experienced an extramedullary CNS relapse. Characteristics of the relapsed patients are demonstrated in Table 2. The relapses occurred 7.9 to 79.5 months after achieving CR. Excluding the 3 patients who experienced extramedullary CNS relapse, the remaining 4 patients received salvage therapy with ATRA±ATO plus GO or IDA, and they all achieved a second CR and underwent stem cell transplantation (2 autologous, 1 matched related donor, 1 haploidentical donor). Three of these patients remain alive in CR. The fourth patient relapsed 3.5 months after autologous stem cell transplant (SCT) and received second salvage therapy with ATRA, ATO, and GO and achieved third CR followed by a matched unrelated donor SCT. He remained alive for 21 months after SCT and died in CR because of pneumococcal meningitis. Among the 3 patients who developed central nervous system relapse, 2 patients had initially experienced cytogenetic and molecular relapse and had received salvage therapy with GO and ATRA/ATO/GO, respectively, after which they both achieved cytogenetic response; 1 patient had molecularly persistent disease, whereas the other achieved molecular CR. Both patients did not receive SCT after second CR and developed CNS relapse treated with ATRA plus intrathecal chemotherapy. One patient achieved CR and underwent matched unrelated donor SCT and remains alive in continuous third CR for 5.2+ years, whereas the other patient failed to respond to treatment and died in CNS relapse. The third patient developed initial isolated CNS relapse treated with multiple lines of salvage therapy, but continued to develop CNS relapse and eventually died as a result of relapsed/refractory APL. With the exception of relapsed patients, all the remaining patients maintained molecular remission with continued follow-up. Seventy-two patients (38.5%) have been followed-up for more than 5 years since study entry.
Characteristics of relapsed acute promyelocytic leukemia patients
| Patient no. . | Risk category . | Age (y) . | Sex . | Cytogenetics . | FLT3 status . | Time to first relapse (mo) . | Type of first relapse . |
|---|---|---|---|---|---|---|---|
| 1 | High | 52 | F | Diploid | ND | 9.2 | Molecular* |
| 2 | Low | 42 | M | 46XY t(15;17) [20] | ND | 79.5 | Hematological/molecular |
| 3 | High | 38 | M | 46XY t(15;17) [19] | ND | 9 | Hematological/molecular |
| 4 | High | 79 | M | 46XY t(15;17), der (17) i (17) (q10) [18]; 46 XY [2] | Neg | 12.4 | Molecular* |
| 5 | High | 18 | M | 46XY t(15;17) [19] | ND | 9.4 | Molecular* |
| 6 | Low | 19 | F | Diploid | Neg | 9.5 | Hematological |
| 7 | High | 35 | M | 46XY t(15;17) [16]; 46 idem, del 7 [1]; 46 XY [3] | Neg | 7.9 | Hematological |
| Patient no. . | Risk category . | Age (y) . | Sex . | Cytogenetics . | FLT3 status . | Time to first relapse (mo) . | Type of first relapse . |
|---|---|---|---|---|---|---|---|
| 1 | High | 52 | F | Diploid | ND | 9.2 | Molecular* |
| 2 | Low | 42 | M | 46XY t(15;17) [20] | ND | 79.5 | Hematological/molecular |
| 3 | High | 38 | M | 46XY t(15;17) [19] | ND | 9 | Hematological/molecular |
| 4 | High | 79 | M | 46XY t(15;17), der (17) i (17) (q10) [18]; 46 XY [2] | Neg | 12.4 | Molecular* |
| 5 | High | 18 | M | 46XY t(15;17) [19] | ND | 9.4 | Molecular* |
| 6 | Low | 19 | F | Diploid | Neg | 9.5 | Hematological |
| 7 | High | 35 | M | 46XY t(15;17) [16]; 46 idem, del 7 [1]; 46 XY [3] | Neg | 7.9 | Hematological |
BM, bone marrow; CNS, central nervous system; F, female; M, Male; MRD-SCT, matched-related donor stem cell transplantation; mo, months; No., number; ND, not done; Neg, negative.
Molecular relapses: Patient No.1 experienced a molecular relapse after 9.2 mo received salvage ATRA/ATO/GO achieved molecular remission, but then had a hematological relapse (BM+CNS) with time to hematological relapse of 18.8 mo (9.6 mo after molecular relapse). Patient No. 4 experienced a molecular relapse after 12.4 mo received salvage GO without achieving molecular remission and had a hematological relapse (CNS alone) with time to hematological relapse of 16.4 mo (4 mo after molecular relapse). Patient No. 5 experienced a molecular relapse after 9.4 mo received salvage ATRA/ATO/GO/IDA achieved molecular remission which was followed by MRD-SCT.
A total of 26 patients died (14%), of whom 7 (4%) died during induction because of disease-related complications, including infection, hemorrhage, and multiorgan failure, with a median time to death of 12 days (range, 7-24 days). Two patients died because of relapsed/refractory APL, including the 1 patient with persistent CNS relapse. The remaining 17 patients died in CR from unrelated causes, including second malignancies (N = 8), sepsis (N = 3), unknown causes (N = 3), renal failure (N = 2), and cardiac failure/arrest (N = 1) (Table 3). Excluding early deaths, the median time to death in the remaining 19 patients was 1224 days (range, 107-3281 days).
Causes of death unrelated to acute promyelocytic leukemia:
| Patient (N = 17) . | Age (y) . | CR duration (mo) . | Cause of death . | Response at time of death . | Comments . |
|---|---|---|---|---|---|
| 1 | 69 | 69.9 | Stage IV GIST | CR | |
| 2 | 77 | 8 | Prostate cancer | CR | |
| 3 | 75 | 60 | Prostate cancer | CR | |
| 4 | 60 | 96.2 | H&N cancer | CR | |
| 5 | 47 | 4.9 | Prostate cancer | CR | |
| 6 | 64 | 77.5 | Melanoma | CR | |
| 7 | 74 | 73.4 | Pancreatic cancer | CR | |
| 8 | 70 | 16.5 | Melanoma | CR | |
| 9 | 69 | 58.4 | ESRD on HD | CR | Renal biopsy showed glomerulosclerosis. Pt had DM. |
| 10 | 75 | 15.7 | ESRD on HD | CR | Received 1 cycle. Therapy discontinued because of fluid overload and worsening creatinine. Pt had other comorbidities including CHF, DM, and HTN. |
| 11 | 38 | 9 | Pneumococcal meningitis | CR | Pt was 644 D post-SCT and was off immunosuppression. Pt had hepatic insufficiency. |
| 12 | 64 | 0.9 | Sepsis/pneumonia | CR | s/p 2 cycles, sepsis unrelated to study drugs. Pt had DM and HTN. |
| 13 | 21 | 9.3 | Infection and pulmonary embolism | CR | Patient was treated with ATRA/ATO/GO on protocol for 2.5 mo and achieved CR but taken off protocol because of ATRA intolerance; ATO/GO off protocol: for 2 mo; IDA off protocol: C3 D59 |
| 14 | 69 | 7 | CHF and cardiac arrest | CR | C3 D31 on protocol. Pt had history of CAD s/p bypass surgery, HTN, and hyperlipidemia. |
| 15 | 81 | 25.2 | Unknown | CR | Lost to follow-up |
| 16 | 81 | 106.8 | Unknown | CR | Lost to follow-up |
| 17 | 43 | 39.2 | Unknown | CR | Lost to follow-up |
| Patient (N = 17) . | Age (y) . | CR duration (mo) . | Cause of death . | Response at time of death . | Comments . |
|---|---|---|---|---|---|
| 1 | 69 | 69.9 | Stage IV GIST | CR | |
| 2 | 77 | 8 | Prostate cancer | CR | |
| 3 | 75 | 60 | Prostate cancer | CR | |
| 4 | 60 | 96.2 | H&N cancer | CR | |
| 5 | 47 | 4.9 | Prostate cancer | CR | |
| 6 | 64 | 77.5 | Melanoma | CR | |
| 7 | 74 | 73.4 | Pancreatic cancer | CR | |
| 8 | 70 | 16.5 | Melanoma | CR | |
| 9 | 69 | 58.4 | ESRD on HD | CR | Renal biopsy showed glomerulosclerosis. Pt had DM. |
| 10 | 75 | 15.7 | ESRD on HD | CR | Received 1 cycle. Therapy discontinued because of fluid overload and worsening creatinine. Pt had other comorbidities including CHF, DM, and HTN. |
| 11 | 38 | 9 | Pneumococcal meningitis | CR | Pt was 644 D post-SCT and was off immunosuppression. Pt had hepatic insufficiency. |
| 12 | 64 | 0.9 | Sepsis/pneumonia | CR | s/p 2 cycles, sepsis unrelated to study drugs. Pt had DM and HTN. |
| 13 | 21 | 9.3 | Infection and pulmonary embolism | CR | Patient was treated with ATRA/ATO/GO on protocol for 2.5 mo and achieved CR but taken off protocol because of ATRA intolerance; ATO/GO off protocol: for 2 mo; IDA off protocol: C3 D59 |
| 14 | 69 | 7 | CHF and cardiac arrest | CR | C3 D31 on protocol. Pt had history of CAD s/p bypass surgery, HTN, and hyperlipidemia. |
| 15 | 81 | 25.2 | Unknown | CR | Lost to follow-up |
| 16 | 81 | 106.8 | Unknown | CR | Lost to follow-up |
| 17 | 43 | 39.2 | Unknown | CR | Lost to follow-up |
C, cycle; CHF, congestive heart failure; D, day; DM, diabetes mellitus; ESRD, end-stage renal disease; GIST, gastrointestinal stromal tumor; HD, hemodialysis: H&N, head and neck; HTN, hypertension; mo, month; Pt, patient; s/p, status post.
Survival
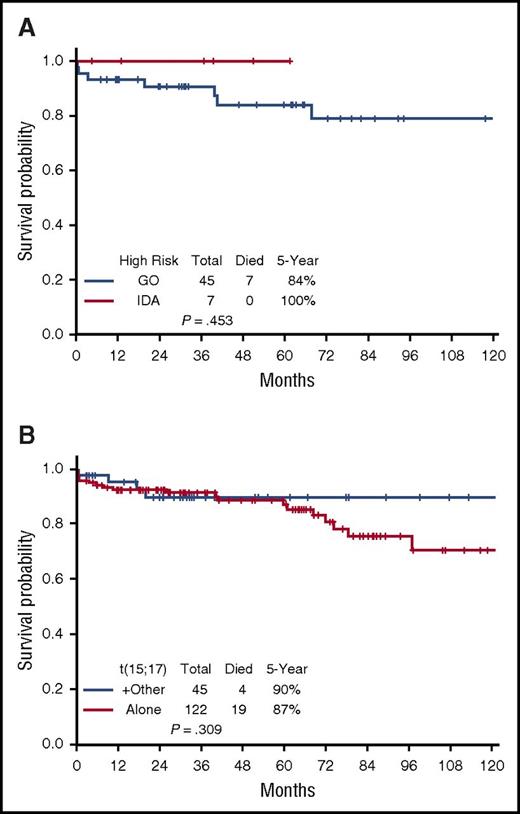
The 5-year EFS, DFS, and OS for the entire cohort are demonstrated in Figure 3A-C, respectively. They are 85%, 96%, and 88%, respectively; the medians have not yet been reached. The EFS, DFS, and OS by risk groups are shown in Figures 4A-C, respectively. The 5-year EFS, DFS, and OS for low-risk patients are 87%, 99% and 89%, respectively, and for the high-risk patients, they are 81%, 89%, and 86%, respectively, indicating that the responses are durable. The 5-year EFS, DFS and OS by age groups are shown in Figures 5A-C, respectively. Among the patients aged 60 years and older, 74% remain alive and disease-free, with a median OS of 97.1+ months. The 5-year OS was similar among the high-risk patients who received GO vs those who received a dose of IDA (Figure 6A). Similarly, there was no difference in OS among the 122 patients with t(15:17) alone detected with standard cytogenetic analysis when compared with the 45 patients who had additional cytogenetic abnormalities (Figure 6B).
Survival outcomes for the whole population. (A) Event-free survival. (B) Disease-free survival. (C) Overall survival for the entire group.
Survival outcomes for the whole population. (A) Event-free survival. (B) Disease-free survival. (C) Overall survival for the entire group.
Outcomes by risk subsets. (A) Event-free survival by risk group. (B) Disease-free survival by risk group. (C) Overall survival by risk group.
Outcomes by risk subsets. (A) Event-free survival by risk group. (B) Disease-free survival by risk group. (C) Overall survival by risk group.
Outcomes by age. (A) Event-free survival by age group. (B) Disease-free survival by age group. (C) Overall survival by age group.
Outcomes by age. (A) Event-free survival by age group. (B) Disease-free survival by age group. (C) Overall survival by age group.
Survival for specified subsets. (A) Gemtuzumab vs idarubicin among high-risk APL patients. (B) Overall survival by cytogenetics.
Survival for specified subsets. (A) Gemtuzumab vs idarubicin among high-risk APL patients. (B) Overall survival by cytogenetics.
Safety and toxicity
Most severe adverse events occurred at induction and were related to the disease and its complications. The most common treatment-related grade 3 and 4 adverse events included infections in 44 patients (23.5%), QT prolongation in 14 patients (7.5%), and hemorrhage in 10 patients (5%). Differentiation syndrome occurred in 21 patients (11%) and was successfully managed in all patients by withholding ATRA and administering corticosteroids, according to standard practice; ATO was not discontinued. Grade 3 to 4 hepatotoxicity was observed in 27 patients (14%).
Discussion
This study further confirms that the combination of ATRA and ATO, with or without GO, is an effective and safe regimen for patients with newly diagnosed APL with durable responses. Only 2 APL-related deaths occurred beyond the first year after study entry, with unrelated malignancies being the major cause of death after the first year. Relapse only occurred in 7 (4%) patients, with the majority of the relapses occurring in the high-risk category (5 patients), and only 3 hematological relapses occurring after the first year.
The combination of ATRA and ATO has been shown to be effective as a frontline treatment of APL.10,22-25 In the randomized study conducted by Shen et al, induction with ATRA plus ATO was effective in achieving CR in 94% of patients and was associated with a shorter remission induction time, enhanced reduction in tumor burden, and lower relapse rates when compared with ATRA or ATO monotherapy.10,35 On long-term follow-up, the 5-year EFS and OS for the ATRA/ATO combination were 89% and 92%, respectively.35 However, all patients in that study received chemotherapy for consolidation. Here, we have demonstrated similar results while eliminating chemotherapy completely.10 Recently, Lo-Coco and colleagues showed superiority of ATRA plus ATO compared with ATRA plus chemotherapy in patients with low- to intermediate-risk APL; they achieved a 2-year EFS of 98% compared with 85% in the chemotherapy group, as well as a statistically significant improvement in OS.23,36
In an attempt to improve outcomes in high-risk patients, we added GO to the ATRA plus ATO combination and reported significant improvement in the CR and survival rates compared with historical reports.24,25 Burnett et al have also conducted a phase 3 randomized controlled, multicenter study (AML17 trial) comparing ATRA, ATO, and GO with ATRA plus chemotherapy in newly diagnosed patients with APL, including the high-risk group.34 They reported superiority of the ATRA, ATO, and GO regimen with a 4-year EFS of 91% compared with 70% in the ATRA plus chemotherapy group. Although the CR and OS rates did not significantly differ between the 2 groups, there was a significant reduction in the supportive care requirements of the ATRA plus ATO group. The 4-year OS for high-risk patients who received ATRA, ATO, and GO was 89%, which is a significant improvement when compared with previous studies. Furthermore, there was a dramatic reduction in the cumulative incidence of morphological and molecular relapse from 18% and 27%, in the ATRA plus chemotherapy group, to 1% and 0%, in the ATRA plus ATO plus GO cohort. These results confirm previously reported data suggesting the synergistic activity of the combination of ATRA and ATO in eradicating the leukemic clone in APL, thereby negating the need for chemotherapy.10,23,25,34 Grade 3 or 4 toxicities occurred more frequently during induction with ATRA plus chemotherapy when compared with ATRA plus ATO, with no evidence of additional liver toxicity or myelosuppression in GO recipients.
The major limitation of our data is its single-group nonrandomized nature, including that patients were only treated at our institution. However, the similarity of outcomes to those in other recently reported randomized studies confirms the utility of this regimen. Compared with our study, the UK investigators used a higher doses of ATO administered less frequently, providing a more convenient schedule and thereby potentially improving compliance without jeopardizing efficacy. Of note, 45% of our low-risk patients received either GO or IDA for leukocytosis, which may have affected our interpretation on durability of response. Furthermore, in the study conducted by Lo-Coco et al, low- to intermediate-risk APL patients who developed leukocytosis were successfully treated with hydroxyurea with no effect on survival when compared with our study.23 This suggests we may be able to manage leukocytosis in these patients using hydroxyurea alone, especially given the lack of GO in many centers. Another important finding in our report is that 52 (22%) of patients with newly diagnosed APL were not included in the trials, including the 13 patients who died within 48 hours of presentation to our institution. This is in concordance with other reports suggesting that early mortality resulting from a delay in diagnosis remains a major obstacle to achieving a close to universal success rate in patients with APL.37 Given that 5 of the 52 high-risk patients with APL who achieved CR relapsed, this raises the question as to whether 1 day of GO or IDA is sufficient to induce durable remissions in this patient population.
Taken together, these findings strongly suggest the feasibility of treatment deescalation, with the complete omission of chemotherapy. This will spare patients the toxicities associated with use of cytotoxic agents with particular benefit for patients unfit for chemotherapy, including older patients, who account for up to 20% of the APL population.7,25,38,39 Such a “chemotherapy-free” regimen will likely reduce the risk for myelosuppression-related complications, as well as cardiotoxicity associated with anthracycline exposure.40 Furthermore, although rare, therapy-related myelodysplastic syndrome and acute myeloid leukemia have been reported with the use of ATRA plus traditional chemotherapy.34,41-43 We have recently reported that the use of ATRA plus ATO is not associated with a higher incidence of secondary malignancies when compared with ATRA plus chemotherapy.44
In conclusion, our long-term data confirm that the chemotherapy-free strategy of ATRA plus ATO, with or without GO, is effective and safe, providing long-term and durable leukemia-free survival for both standard-risk and high-risk patients, with rare relapses after the first year. In addition to these data, the results published by Burnett et al are sufficient evidence that the combination of ATRA, ATO, and GO is at least as effective as ATRA plus chemotherapy in high-risk patients, while providing a more tolerable regimen. Therefore, the combination of ATRA and ATO, with or without GO, should be considered as the new standard of care for all newly diagnosed patients with APL, and efforts to reinstate the availability of GO should be encouraged.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
Gemtuzumab ozogamicin was provided by Pfizer.
Authorship
Contribution: Conception and design: F.R., E.E., H.K.; provision of study materials or patients: F.R., E.E., G.G.-M., G.B., E.J., S.F., S.O., W.W., S.K., T.K., N.D., C.D., S.V., A.F., M.A., M.K., Z.E., J.C., H.K., R.L., K.P., N.J.; collection and assembly of data: F.R., M.F., M.B., S.P., Y.A., David McCue; data analysis and interpretation: F.R., E.E., S.P., H.K., Y.A., M.B., Deborah McCue; manuscript writing: Y.A., F.R.; and final approval of manuscript: F.R., E.E., H.K., and all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Department of Leukemia, University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.