Key Points
An international panel established the first ever diagnostic criteria for iMCD based on review of 244 clinical cases and 88 tissue samples.
The criteria require multicentric lymphadenopathy with defined histopathology, ≥2 clinical/laboratory changes, and exclusion of iMCD mimics.
Abstract
Human herpesvirus-8 (HHV-8)–negative, idiopathic multicentric Castleman disease (iMCD) is a rare and life-threatening disorder involving systemic inflammatory symptoms, polyclonal lymphoproliferation, cytopenias, and multiple organ system dysfunction caused by a cytokine storm often including interleukin-6. iMCD accounts for one third to one half of all cases of MCD and can occur in individuals of any age. Accurate diagnosis is challenging, because no standard diagnostic criteria or diagnostic biomarkers currently exist, and there is significant overlap with malignant, autoimmune, and infectious disorders. An international working group comprising 34 pediatric and adult pathology and clinical experts in iMCD and related disorders from 8 countries, including 2 physicians that are also iMCD patients, was convened to establish iMCD diagnostic criteria. The working group reviewed data from 244 cases, met twice, and refined criteria over 15 months (June 2015 to September 2016). The proposed consensus criteria require both Major Criteria (characteristic lymph node histopathology and multicentric lymphadenopathy), at least 2 of 11 Minor Criteria with at least 1 laboratory abnormality, and exclusion of infectious, malignant, and autoimmune disorders that can mimic iMCD. Characteristic histopathologic features may include a constellation of regressed or hyperplastic germinal centers, follicular dendritic cell prominence, hypervascularization, and polytypic plasmacytosis. Laboratory and clinical Minor Criteria include elevated C-reactive protein or erythrocyte sedimentation rate, anemia, thrombocytopenia or thrombocytosis, hypoalbuminemia, renal dysfunction or proteinuria, polyclonal hypergammaglobulinemia, constitutional symptoms, hepatosplenomegaly, effusions or edema, eruptive cherry hemangiomatosis or violaceous papules, and lymphocytic interstitial pneumonitis. iMCD consensus diagnostic criteria will facilitate consistent diagnosis, appropriate treatment, and collaborative research.
Introduction
Castleman disease (CD) encompasses several clinicopathologic disorders at the intersection of hematology, oncology, rheumatology, and virology, with overlap in histopathologic and clinical features. Historically, CD has been classified as unicentric or multicentric. A subset of multicentric CD (MCD) is caused by human herpesvirus-8 (HHV-8; also known as Kaposi sarcoma–associated herpesvirus) (HHV-8-associated MCD), whereas HHV-8–negative MCD cases remain idiopathic (iMCD). Unicentric CD (UCD) involves a single lymph node region showing characteristic “Castleman-like” histopathologic changes.1-3 Inflammatory manifestations are generally mild in UCD and usually disappear after surgical excision of the lymph node.1 In contrast, both iMCD and HHV-8–associated MCD are characterized by multifocal lymphadenopathy with a range of histopathology and episodic systemic inflammatory symptoms. HHV-8–associated MCD is most commonly diagnosed in HIV-infected or otherwise immunocompromised individuals. Virally-encoded interleukin (IL)-6 and human IL-6 are implicated in disease pathogenesis.4-8
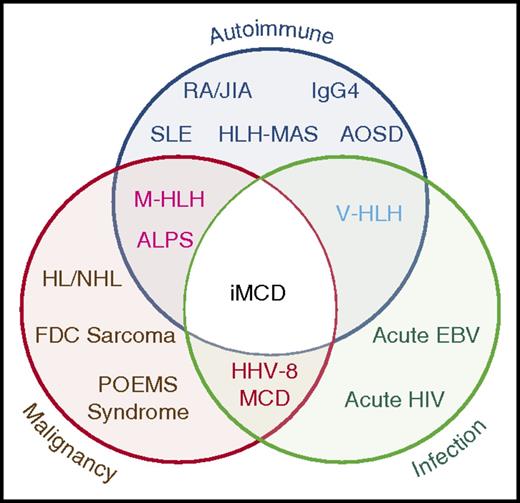
HHV-8–negative/iMCD is less well understood and has no specific biomarkers. Currently, iMCD is diagnosed when a constellation of nonspecific but characteristic lymph node histopathologic features commonly described as “hyaline vascular,” “plasmacytic,” or “mixed” are observed in patients with appropriate clinical features.9,10 HHV-8–associated MCD and a range of malignant, autoimmune, and infectious disorders known to mimic these features (Figure 1) should be excluded.11 The etiology of iMCD is unknown, although it is hypothesized to involve one or more of the following mechanisms: autoimmunity/autoinflammation (ie, pathologic auto-antibodies or germline genomic alterations in inflammatory pathways); paraneoplastic (ie, somatic mutations in clonal cells); or infection with a virus other than HHV-8.12 It is possible that multiple pathways culminate in a cytokine storm that results in similar clinical presentations.
Significant clinical, histologic, and immunologic overlap between iMCD, malignancy, autoimmune, and infectious disorders. The exact location for iMCD on the spectrum from autoimmune, malignant, and infectious diseases is currently unknown and may vary from patient to patient. ALPS, autoimmune lymphoproliferative syndrome; AOSD, adult-onset Still disease; EBV, Epstein-Barr virus; FDC, follicular dendritic cell; HHV-8, human herpesvirus-8; HL, Hodgkin lymphoma; HLH-MAS, hemophagocytic lymphohistiocytosis- macrophage activation syndrome; IgG4, IgG4-related disease; JIA, juvenile idiopathic arthritis; M-HLH, malignancy-associated hemophagocytic lymphohistiocytosis; NHL, non-Hodgkin lymphoma; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal paraprotein, skin changes; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; V-HLH, viral hemophagocytic lymphohistiocytosis.
Significant clinical, histologic, and immunologic overlap between iMCD, malignancy, autoimmune, and infectious disorders. The exact location for iMCD on the spectrum from autoimmune, malignant, and infectious diseases is currently unknown and may vary from patient to patient. ALPS, autoimmune lymphoproliferative syndrome; AOSD, adult-onset Still disease; EBV, Epstein-Barr virus; FDC, follicular dendritic cell; HHV-8, human herpesvirus-8; HL, Hodgkin lymphoma; HLH-MAS, hemophagocytic lymphohistiocytosis- macrophage activation syndrome; IgG4, IgG4-related disease; JIA, juvenile idiopathic arthritis; M-HLH, malignancy-associated hemophagocytic lymphohistiocytosis; NHL, non-Hodgkin lymphoma; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal paraprotein, skin changes; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; V-HLH, viral hemophagocytic lymphohistiocytosis.
iMCD patients experience systemic inflammation, polyclonal lymphoproliferation, and a wide spectrum of symptoms caused by a cytokine storm often including IL-6 and vascular endothelial growth factor (VEGF).11-13 Clinical hallmarks include fever, night sweats, lymphadenopathy, ascites, hepatosplenomegaly, elevated C-reactive protein (CRP), hypoalbuminemia, and anemia.12 Some patients experience mild flulike symptoms, whereas others experience severe sepsislike multiple organ system failure, anasarca, and death.
The recently described “TAFRO syndrome” identifies a subset of iMCD patients with shared manifestations, including thrombocytopenia, anasarca/ascites, reticulin fibrosis in bone marrow, renal dysfunction, organomegaly (TAFRO), and typically normal immunoglobulin levels.14 Although first described in Japan in 2010,15 iMCD patients with TAFRO features have been observed around the world for decades.11,14,16-20 iMCD patients without TAFRO syndrome typically have thrombocytosis, hypergammaglobulinemia, and less severe fluid accumulation. This non-TAFRO group has been called idiopathic plasmacytic lymphadenopathy with polyclonal hyperimmunoglobulinemia or IPL-type.14
There are an estimated 6500 to 7700 new CD cases diagnosed/year in the United States, with ∼1650 cases of MCD.21 iMCD accounts for 33% to 58% of published MCD cases.11 iMCD can occur in individuals of any age with a range of 2 to 80 years (median, 50).11 Historically, 35% die within 5 years of diagnosis, 60% die within 10 years,22 and patients have a threefold increased prevalence of malignancy.11 Corticosteroids, rituximab, cytotoxic chemotherapy, immunosuppressants, immunomodulators, and anti–IL-6 therapies have all been reported for the treatment of iMCD.11 Antibodies targeting IL-6 (siltuximab)23 or the IL-6 co-receptor, gp80 (tocilizumab),24,25 can reverse symptoms in many patients and may improve long-term outcomes. Siltuximab was recently approved for iMCD based on results from an international randomized, controlled trial in which 34% of patients attained a complete or partial response compared with 0% on placebo.23,26 However, the lack of defined diagnostic criteria or disease-specific biomarkers can impede timely administration of treatment before organ dysfunction and death may occur. Clinicopathologic diagnostic criteria are urgently needed to facilitate timely recognition, diagnostic workup, and research into pathogenesis and treatment. In this study, we present a multidisciplinary, evidence-based consensus diagnostic criteria for iMCD.
Methods
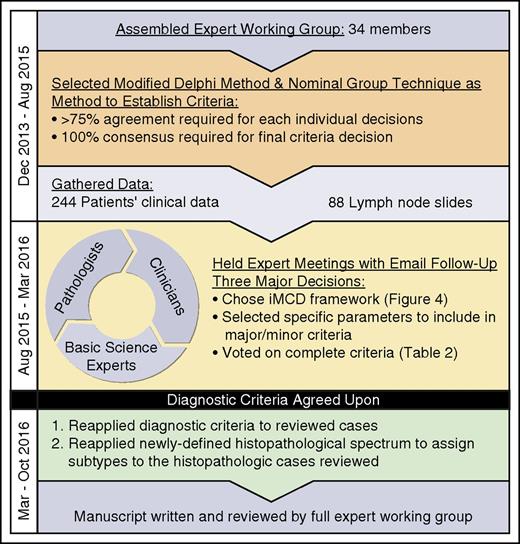
In 2013, the Castleman Disease Collaborative Network (CDCN)27 Scientific Advisory Board prioritized the establishment of an evidence-based, patient-guided, expert consensus diagnostic criteria. An international working group comprising 34 pediatric and adult hematopathology, hematology/oncology, rheumatology, immunology, and infectious diseases experts in iMCD and related disorders representing 8 countries on 5 continents, including 2 physicians that are also iMCD patients, was assembled (Figure 2). The CDCN assembled clinical data for 244 iMCD patients as well as 88 lymph node tissue biopsies for histopathologic review. One-hundred twenty-eight cases came from a systematic literature review of pathology-based iMCD, where HHV-8 was excluded and individual clinical data were available,11 37 cases were submitted by working group members, and 79 were from a randomized controlled study of siltuximab in subjects with symptomatic iMCD (NCT01024036).23 Cases with <80 k/µL platelets, elevated transaminases, and/or kidney dysfunction were excluded from NCT01024036, and (46/79) 58% of included cases received treatment before enrollment.23
Process of criteria development. An international working group with 34 leading physicians, pathologists, and clinicians was created to develop the diagnostic criteria for iMCD. A modified Delphi Method & Nominal Group Technique was selected to guide the criteria development process. A total of 244 patients’ clinical data were gathered along with lymph node slides from 88 cases. Two working group meetings were held to establish an agreed-upon diagnostic criteria. Postmeeting analyses were performed to reapply the agreed-upon diagnostic criteria to 79 cases from NCT01024036 and to use the newly defined histopathologic spectrum to subtype cases. The consensus criteria and results from analyses were compiled into a manuscript that was reviewed by the full expert working group.
Process of criteria development. An international working group with 34 leading physicians, pathologists, and clinicians was created to develop the diagnostic criteria for iMCD. A modified Delphi Method & Nominal Group Technique was selected to guide the criteria development process. A total of 244 patients’ clinical data were gathered along with lymph node slides from 88 cases. Two working group meetings were held to establish an agreed-upon diagnostic criteria. Postmeeting analyses were performed to reapply the agreed-upon diagnostic criteria to 79 cases from NCT01024036 and to use the newly defined histopathologic spectrum to subtype cases. The consensus criteria and results from analyses were compiled into a manuscript that was reviewed by the full expert working group.
An international symposium sponsored by the CDCN and University of Pennsylvania Orphan Disease Center was held on November 20-21, 2015 in Philadelphia, Pennsylvania with 21 expert participants, and a follow-up meeting was held on December 6, 2015 in Orlando, Florida with 19 participants. All votes were anonymous and >75% agreement was needed to pass an individual decision. The final criteria vote required 100% consensus.
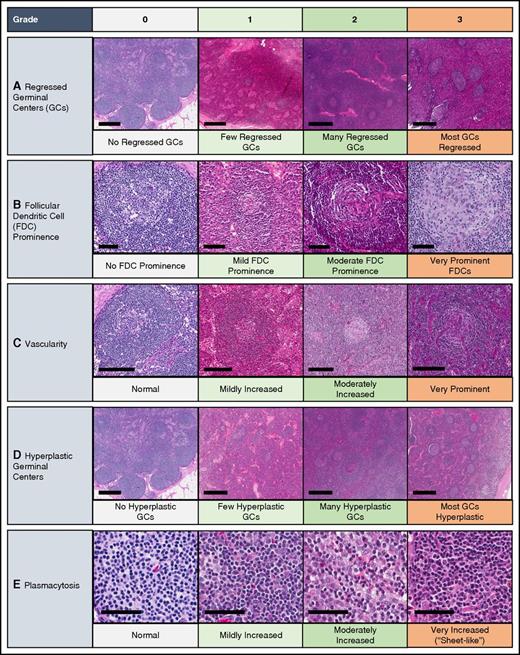
Literature reviews and expert interviews were performed to select a hybrid Delphi method and Nominal Group Technique (NGT) approach28 to guide criteria development. Clinical and laboratory parameters were chosen for consideration from literature review and expert nomination via the Delphi method in advance of the meetings (Table 1). NGT was used during the meetings to select parameters through group discussion and secret ballots and to achieve consensus. A team of expert hematopathologists examined hematoxylin and eosin–stained lymph node slides from 88 cases with a presumptive diagnosis of iMCD and graded the following histopathologic features using a scale of 0-3: regressed germinal centers (GCs), follicular dendritic cell (FDC) prominence, vascular proliferation, plasmacytosis, and hyperplastic GCs (Figure 3). The team expanded during the working group meeting to include additional hematopathologists. The group reviewed each case simultaneously at a multihead microscope until a majority of reviewers voted on a grade for each feature. The average grade for each histopathologic feature assigned during review was calculated and compared between subtypes by 2-way analysis of variance using a generalized linear model. Three of the 88 submitted pathology cases had insufficient tissue to be fully assessed.
Subset of clinical and laboratory features considered in determining diagnostic criteria
| . | Systematic literature review cases (N = 128)11 . | Siltuximab trial cases (N = 79)23 . | Submitted cases (N = 37) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical features . | Reported positive finding . | Reported positive or negative finding . | % of patients, minimum* . | % of patients, maximum† . | Reported positive finding . | Reported positive or negative finding . | % of patients, minimum* . | % of patients, maximum† . | Reported positive finding . | Reported positive or negative finding . | % of patients, maximum* . |
| Multicentric lymphadenopathy | 128 | 128 | 100 | 100 | 37 | 37 | 100 | ||||
| Constitutional symptoms | |||||||||||
| Fatigue | 68 | 79 | 86 | 86 | 3 | 3 | 100 | ||||
| Night sweats | 13 | 21 | 10 | 62 | 41 | 79 | 52 | 52 | 1 | 1 | 100 |
| Weight loss | 21 | 29 | 16 | 72 | 24 | 79 | 30 | 30 | |||
| Fever | 33 | 64 | 26 | 52 | 13 | 79 | 17 | 17 | 25 | 37 | 68 |
| Enlarged liver ± spleen (per CT) | 52 | 67 | 41 | 78 | 10 | 14 | 71 | ||||
| Palpable liver | 10 | 79 | 13 | 13 | 1 | 2 | 50 | ||||
| Palpable spleen or splenomegaly | 9 | 79 | 11 | 11 | 11 | 24 | 46 | ||||
| Edema, ascites, ± anasarca‡ | 29 | 37 | 23 | 78 | 34 | 79 | 43 | 43 | 23 | 37 | 62 |
| Laboratory features | |||||||||||
| Low hemoglobin§ | 79 | 91 | 62 | 87 | 32 | 59 | 41 | 54 | 28 | 37 | 76 |
| Thrombocytopenia‖ | 28 | 63 | 22 | 44 | 5 | 59 | 6 | 8 | 13 | 15 | 87 |
| Thrombocytosis¶ | 16 | 63 | 13 | 25 | 15 | 59 | 19 | 25 | 1 | 15 | 7 |
| Elevated ESR# | 44 | 48 | 34 | 92 | 35 | 52 | 44 | 67 | |||
| Elevated CRP** | 65 | 79 | 51 | 82 | 41 | 79 | 49 | 52 | 31 | 37 | 84 |
| Elevated sIL-2R†† | 20 | 21 | 16 | 95 | 6 | 12 | 50 | ||||
| Elevated VEGF‡‡ | 16 | 20 | 13 | 80 | 7 | 9 | 78 | ||||
| Elevated IL-6a | 57 | 63 | 45 | 90 | 13 | 75 | 17 | 17 | 12 | 12 | 100 |
| Elevated IgG levelsb | 63 | 82 | 49 | 77 | 36 | 79 | 46 | 46 | 19 | 37 | 51 |
| Elevated IgA levelsc | 32 | 79 | 41 | 41 | |||||||
| Hypoalbuminemiad | 57 | 63 | 45 | 90 | 24 | 57 | 30 | 42 | 25 | 37 | 68 |
| Renal dysfunctione | 12 | 17 | 9 | 71 | 5 | 57 | 6 | 9 | 9 | 15 | 60 |
| . | Systematic literature review cases (N = 128)11 . | Siltuximab trial cases (N = 79)23 . | Submitted cases (N = 37) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical features . | Reported positive finding . | Reported positive or negative finding . | % of patients, minimum* . | % of patients, maximum† . | Reported positive finding . | Reported positive or negative finding . | % of patients, minimum* . | % of patients, maximum† . | Reported positive finding . | Reported positive or negative finding . | % of patients, maximum* . |
| Multicentric lymphadenopathy | 128 | 128 | 100 | 100 | 37 | 37 | 100 | ||||
| Constitutional symptoms | |||||||||||
| Fatigue | 68 | 79 | 86 | 86 | 3 | 3 | 100 | ||||
| Night sweats | 13 | 21 | 10 | 62 | 41 | 79 | 52 | 52 | 1 | 1 | 100 |
| Weight loss | 21 | 29 | 16 | 72 | 24 | 79 | 30 | 30 | |||
| Fever | 33 | 64 | 26 | 52 | 13 | 79 | 17 | 17 | 25 | 37 | 68 |
| Enlarged liver ± spleen (per CT) | 52 | 67 | 41 | 78 | 10 | 14 | 71 | ||||
| Palpable liver | 10 | 79 | 13 | 13 | 1 | 2 | 50 | ||||
| Palpable spleen or splenomegaly | 9 | 79 | 11 | 11 | 11 | 24 | 46 | ||||
| Edema, ascites, ± anasarca‡ | 29 | 37 | 23 | 78 | 34 | 79 | 43 | 43 | 23 | 37 | 62 |
| Laboratory features | |||||||||||
| Low hemoglobin§ | 79 | 91 | 62 | 87 | 32 | 59 | 41 | 54 | 28 | 37 | 76 |
| Thrombocytopenia‖ | 28 | 63 | 22 | 44 | 5 | 59 | 6 | 8 | 13 | 15 | 87 |
| Thrombocytosis¶ | 16 | 63 | 13 | 25 | 15 | 59 | 19 | 25 | 1 | 15 | 7 |
| Elevated ESR# | 44 | 48 | 34 | 92 | 35 | 52 | 44 | 67 | |||
| Elevated CRP** | 65 | 79 | 51 | 82 | 41 | 79 | 49 | 52 | 31 | 37 | 84 |
| Elevated sIL-2R†† | 20 | 21 | 16 | 95 | 6 | 12 | 50 | ||||
| Elevated VEGF‡‡ | 16 | 20 | 13 | 80 | 7 | 9 | 78 | ||||
| Elevated IL-6a | 57 | 63 | 45 | 90 | 13 | 75 | 17 | 17 | 12 | 12 | 100 |
| Elevated IgG levelsb | 63 | 82 | 49 | 77 | 36 | 79 | 46 | 46 | 19 | 37 | 51 |
| Elevated IgA levelsc | 32 | 79 | 41 | 41 | |||||||
| Hypoalbuminemiad | 57 | 63 | 45 | 90 | 24 | 57 | 30 | 42 | 25 | 37 | 68 |
| Renal dysfunctione | 12 | 17 | 9 | 71 | 5 | 57 | 6 | 9 | 9 | 15 | 60 |
BUN, blood urea nitrogen; CRP, C-reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; IL-6, interleukin-6; sIL-2R, soluble interleukin-2 receptor; VEGF, vascular endothelial growth factor.
Positive cases divided by the number of total cases.
Positive cases divided by reported positive and negative findings.
For siltuximab trial patients, definition of “fluid retention” was used.
For systematic review patients: hemoglobin <115 g/L or stated “anemic”; for siltuximab trial patients, hemoglobin less than stated reference range; and for submitted cases patients, hemoglobin <125 g/L.
For systematic review patients, platelet count <150 × 109/L or stated “thrombocytopenia”; for siltuximab trial patients, platelet count less than stated reference range; and for submitted cases patients, platelet count <150 × 109/L.
For systematic review patients, platelet count >500 × 109/L or stated “thrombocytosis”; for siltuximab trial patients, platelet count greater than stated reference range; and for submitted cases patients, platelet count >500 × 109/L.
For systematic review patients, ESR >30 mm/h or stated “elevated ESR”; and for siltuximab trial patients, ESR greater than stated reference range.
For systematic review patients, CRP >10 mg/L or CRP >95.24 nmol/L; for siltuximab trial patients, CRP greater than stated reference range; and for submitted cases patients, CRP >10 mg/L.
For systematic review patients, sIL-2R greater than stated reference range; and for submitted cases patients, sIL-2R greater than stated reference range.
For systematic review patients, VEGF >100 pg/mL or VEGF greater than stated reference range; and for submitted cases patients, VEGF greater than stated reference range.
For systematic review patients, IL-6 >6 pg/mL or IL-6 greater than stated reference range; for siltuximab trial patients, IL-6 greater than stated reference range; and for submitted cases patients, IL-6 greater than stated reference range.
For systematic review patients, IgG >1700 mg/dL or stated “hypergammaglobulinemia”; for siltuximab trial patients, IgG greater than stated reference range; and for submitted cases patients, IgG >17 000 g/L.
For siltuximab trial patients, IgA greater than stated reference range.
For systematic review patients, albumin <3.5 g/dL; for siltuximab trial patients, albumin less than stated reference range; and for submitted cases patients, albumin <35 g/L.
For systematic review patients, creatinine >106 μmol/L or BUN >7.14 mmol/L; for siltuximab trial patients, creatinine greater than stated reference range; and for submitted cases patients, creatinine >106 μmol/L or greater than stated reference range, or BUN >7.14 mmol/L.
Grading of pathologic features seen in iMCD. The following images are examples of the respective grades for each histopathologic feature. Deidentified lymph node slides were obtained prestained with hematoxylin and eosin from Janssen Pharmaceuticals and scanned using an Aperio CS scanner (Leica Biosystems, Wetzlar, Germany) at 20×/0.75NA Plan Apochromat. Images were captured using Aperio Imagescope and enhanced to 300 dpi using Photoshop. Bars represent 500 μm (A,D), 80 μm (B), 200 μm (C), 60 μm (E). (A) Regressed/atrophic germinal centers. (B) Follicular dendritic cell prominence. (C) Vascularity. (D) Hyperplastic germinal centers. (E) Plasmacytosis.
Grading of pathologic features seen in iMCD. The following images are examples of the respective grades for each histopathologic feature. Deidentified lymph node slides were obtained prestained with hematoxylin and eosin from Janssen Pharmaceuticals and scanned using an Aperio CS scanner (Leica Biosystems, Wetzlar, Germany) at 20×/0.75NA Plan Apochromat. Images were captured using Aperio Imagescope and enhanced to 300 dpi using Photoshop. Bars represent 500 μm (A,D), 80 μm (B), 200 μm (C), 60 μm (E). (A) Regressed/atrophic germinal centers. (B) Follicular dendritic cell prominence. (C) Vascularity. (D) Hyperplastic germinal centers. (E) Plasmacytosis.
At the conclusion of the meetings, the newly established diagnostic criteria were applied separately to cases that met both Major Criteria from the literature review, submitted cases, and NCT01024036, to evaluate the number of reported Minor Criteria required for the case definition. We also calculated response to siltuximab in NCT01024036 based on the number of Minor Criteria. We calculated summary statistics by tabulation and percentages. Fisher’s exact test was used to assess significant differences in treatment response rate. A value of P < .05 was considered significant. Statistical tests were performed using SAS 9.4.
Results
Members of the working group first discussed the scope of an iMCD diagnosis and voted unanimously in favor of using the iMCD definition, depicted in Figure 4. The framework highlights that “Castleman-like” features can be observed in multiple regions of enlarged lymph nodes in 4 settings: diseases other than MCD (ie, diseases to exclude), HHV-8–negative MCD associated with POEMS syndrome, iMCD (HHV-8–negative MCD without POEMS), and HHV-8–associated MCD. There was agreement to distinguish MCD patients with POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes) syndrome from iMCD, because POEMS is associated with a monoclonal plasma cell disorder and has a different natural history and therapeutic approach from iMCD.
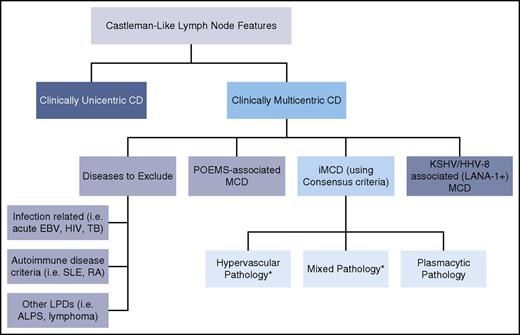
Algorithmic approach for assessment of lymph node with features of CD. Patients with lymph nodes with histologic features suggestive of CD should be evaluated for sites of involvement. If lymph node involvement is restricted to one site, the lesion most likely represents unicentric CD. If multiple sites are involved, patients should be evaluated for HHV-8, POEMS, and other infectious, malignant, and autoimmune disorders listed in Table 2 Exclusion Criteria. If these conditions are excluded, a diagnosis of iMCD should be considered. There are 3 major histopathologic subtypes of iMCD: hypervascular (formerly hyaline-vascular), mixed, and plasmacytic pathology. *iMCD patients with TAFRO syndrome frequently demonstrate hypervascular or mixed pathology.
Algorithmic approach for assessment of lymph node with features of CD. Patients with lymph nodes with histologic features suggestive of CD should be evaluated for sites of involvement. If lymph node involvement is restricted to one site, the lesion most likely represents unicentric CD. If multiple sites are involved, patients should be evaluated for HHV-8, POEMS, and other infectious, malignant, and autoimmune disorders listed in Table 2 Exclusion Criteria. If these conditions are excluded, a diagnosis of iMCD should be considered. There are 3 major histopathologic subtypes of iMCD: hypervascular (formerly hyaline-vascular), mixed, and plasmacytic pathology. *iMCD patients with TAFRO syndrome frequently demonstrate hypervascular or mixed pathology.
The 3-part criteria in Table 2 were unanimously accepted by the working group. To diagnose iMCD, a patient must meet both Major Criteria, have at least 2 of 11 Minor Criteria including at least 1 laboratory abnormality, and have diseases listed in the Exclusion Criteria ruled out.
Consensus diagnostic criteria for iMCD
| I. Major Criteria (need both): |
| 1. Histopathologic lymph node features consistent with the iMCD spectrum (Figure 5). Features along the iMCD spectrum include (need grade 2-3 for either regressive GCs or plasmacytosis at minimum): |
| Regressed/atrophic/atretic germinal centers, often with expanded mantle zones composed of concentric rings of lymphocytes in an “onion skinning” appearance |
| FDC prominence |
| Vascularity, often with prominent endothelium in the interfollicluar space and vessels penetrating into the GCs with a “lollipop” appearance |
| Sheetlike, polytypic plasmacytosis in the interfollicular space |
| Hyperplastic GCs |
| 2. Enlarged lymph nodes (≥1 cm in short-axis diameter) in ≥2 lymph node stations |
| II. Minor Criteria (need at least 2 of 11 criteria with at least 1 laboratory criterion) |
| Laboratory* |
| 1. Elevated CRP (>10 mg/L) or ESR (>15 mm/h)† |
| 2. Anemia (hemoglobin <12.5 g/dL for males, hemoglobin <11.5 g/dL for females) |
| 3. Thrombocytopenia (platelet count <150 k/μL) or thrombocytosis (platelet count >400 k/μL) |
| 4. Hypoalbuminemia (albumin <3.5 g/dL) |
| 5. Renal dysfunction (eGFR <60 mL/min/1.73m2) or proteinuria (total protein 150 mg/24 h or 10 mg/100 ml) |
| 6. Polyclonal hypergammaglobulinemia (total γ globulin or immunoglobulin G >1700 mg/dL) |
| Clinical |
| 1. Constitutional symptoms: night sweats, fever (>38°C), weight loss, or fatigue (≥2 CTCAE lymphoma score for B-symptoms) |
| 2. Large spleen and/or liver |
| 3. Fluid accumulation: edema, anasarca, ascites, or pleural effusion |
| 4. Eruptive cherry hemangiomatosis or violaceous papules |
| 5. Lymphocytic interstitial pneumonitis |
| III. Exclusion Criteria (must rule out each of these diseases that can mimic iMCD) |
| Infection-related disorders |
| 1. HHV-8 (infection can be documented by blood PCR, diagnosis of HHV-8–associated MCD requires positive LANA-1 staining by IHC, which excludes iMCD) |
| 2. Clinical EBV-lymphoproliferative disorders such as infectious mononucleosis or chronic active EBV (detectable EBV viral load not necessarily exclusionary) |
| 3. Inflammation and adenopathy caused by other uncontrolled infections (eg, acute or uncontrolled CMV, toxoplasmosis, HIV, active tuberculosis) |
| Autoimmune/autoinflammatory diseases (requires full clinical criteria, detection of autoimmune antibodies alone is not exclusionary) |
| 1. Systemic lupus erythematosus |
| 2. Rheumatoid arthritis |
| 3. Adult-onset Still disease |
| 4. Juvenile idiopathic arthritis |
| 5. Autoimmune lymphoproliferative syndrome |
| Malignant/lymphoproliferative disorders (these disorders must be diagnosed before or at the same time as iMCD to be exclusionary): |
| 1. Lymphoma (Hodgkin and non-Hodgkin) |
| 2. Multiple myeloma |
| 3. Primary lymph node plasmacytoma |
| 4. FDC sarcoma |
| 5. POEMS syndrome‡ |
| Select additional features supportive of, but not required for diagnosis |
| Elevated IL-6, sIL-2R, VEGF, IgA, IgE, LDH, and/or B2M |
| Reticulin fibrosis of bone marrow (particularly in patients with TAFRO syndrome) |
| Diagnosis of disorders that have been associated with iMCD: paraneoplastic pemphigus, bronchiolitis obliterans organizing pneumonia, autoimmune cytopenias, polyneuropathy (without diagnosing POEMS‡), glomerular nephropathy, inflammatory myofibroblastic tumor |
| I. Major Criteria (need both): |
| 1. Histopathologic lymph node features consistent with the iMCD spectrum (Figure 5). Features along the iMCD spectrum include (need grade 2-3 for either regressive GCs or plasmacytosis at minimum): |
| Regressed/atrophic/atretic germinal centers, often with expanded mantle zones composed of concentric rings of lymphocytes in an “onion skinning” appearance |
| FDC prominence |
| Vascularity, often with prominent endothelium in the interfollicluar space and vessels penetrating into the GCs with a “lollipop” appearance |
| Sheetlike, polytypic plasmacytosis in the interfollicular space |
| Hyperplastic GCs |
| 2. Enlarged lymph nodes (≥1 cm in short-axis diameter) in ≥2 lymph node stations |
| II. Minor Criteria (need at least 2 of 11 criteria with at least 1 laboratory criterion) |
| Laboratory* |
| 1. Elevated CRP (>10 mg/L) or ESR (>15 mm/h)† |
| 2. Anemia (hemoglobin <12.5 g/dL for males, hemoglobin <11.5 g/dL for females) |
| 3. Thrombocytopenia (platelet count <150 k/μL) or thrombocytosis (platelet count >400 k/μL) |
| 4. Hypoalbuminemia (albumin <3.5 g/dL) |
| 5. Renal dysfunction (eGFR <60 mL/min/1.73m2) or proteinuria (total protein 150 mg/24 h or 10 mg/100 ml) |
| 6. Polyclonal hypergammaglobulinemia (total γ globulin or immunoglobulin G >1700 mg/dL) |
| Clinical |
| 1. Constitutional symptoms: night sweats, fever (>38°C), weight loss, or fatigue (≥2 CTCAE lymphoma score for B-symptoms) |
| 2. Large spleen and/or liver |
| 3. Fluid accumulation: edema, anasarca, ascites, or pleural effusion |
| 4. Eruptive cherry hemangiomatosis or violaceous papules |
| 5. Lymphocytic interstitial pneumonitis |
| III. Exclusion Criteria (must rule out each of these diseases that can mimic iMCD) |
| Infection-related disorders |
| 1. HHV-8 (infection can be documented by blood PCR, diagnosis of HHV-8–associated MCD requires positive LANA-1 staining by IHC, which excludes iMCD) |
| 2. Clinical EBV-lymphoproliferative disorders such as infectious mononucleosis or chronic active EBV (detectable EBV viral load not necessarily exclusionary) |
| 3. Inflammation and adenopathy caused by other uncontrolled infections (eg, acute or uncontrolled CMV, toxoplasmosis, HIV, active tuberculosis) |
| Autoimmune/autoinflammatory diseases (requires full clinical criteria, detection of autoimmune antibodies alone is not exclusionary) |
| 1. Systemic lupus erythematosus |
| 2. Rheumatoid arthritis |
| 3. Adult-onset Still disease |
| 4. Juvenile idiopathic arthritis |
| 5. Autoimmune lymphoproliferative syndrome |
| Malignant/lymphoproliferative disorders (these disorders must be diagnosed before or at the same time as iMCD to be exclusionary): |
| 1. Lymphoma (Hodgkin and non-Hodgkin) |
| 2. Multiple myeloma |
| 3. Primary lymph node plasmacytoma |
| 4. FDC sarcoma |
| 5. POEMS syndrome‡ |
| Select additional features supportive of, but not required for diagnosis |
| Elevated IL-6, sIL-2R, VEGF, IgA, IgE, LDH, and/or B2M |
| Reticulin fibrosis of bone marrow (particularly in patients with TAFRO syndrome) |
| Diagnosis of disorders that have been associated with iMCD: paraneoplastic pemphigus, bronchiolitis obliterans organizing pneumonia, autoimmune cytopenias, polyneuropathy (without diagnosing POEMS‡), glomerular nephropathy, inflammatory myofibroblastic tumor |
B2M, β-2-microglobulin; CMV, cytomegalovirus; CTCAE, common terminology for adverse events; eGFR, estimated glomerular filtration rate; GC, germinal center; IHC, Immunohistochemistry; LANA-1, latency-associated nuclear antigen; LDH, lactate dehydrogenase.
We have provided laboratory cutoff thresholds as guidance, but we recognize that some laboratories have slightly different ranges. We suggest that you use the upper and lower ranges from your particular laboratory to determine if a patient meets a particular laboratory Minor Criterion.
Evaluation of CRP is mandatory and tracking CRP levels is highly recommended, but ESR will be accepted if CRP is not available.
POEMS is considered to be a disease “associated” with CD. Because the monoclonal plasma cells are believed to drive the cytokine storm, we do not consider it iMCD, but rather “POEMS-associated MCD.”
Major Criteria
Major Criterion 1 requires histopathologic features consistent with iMCD on an excisional lymph node biopsy. After extensive histologic review and discussion, the group voted in favor of defining the 2 ends of the histologic spectrum as well as cases with “mixed” characteristics in between these 2 ends that would be compatible with a diagnosis of iMCD and meet Major Criterion 1 (Figure 5). To satisfy Major Criterion 1, patients need a grade 2 or 3 for regressed GCs or plasmacytosis as well as other features consistent with the iMCD histologic spectrum. Using the established criteria based on consensus discussions, 71 of 85 cases with sufficient tissue exemplified the newly accepted histopathologic criteria, which included 63 of 76 patients from NCT01024036. After the meeting, these cases were re-reviewed to classify them into 1 of 3 subtypes defined during the meeting, and the scoring of particular features were assessed for each group (Figure 6).
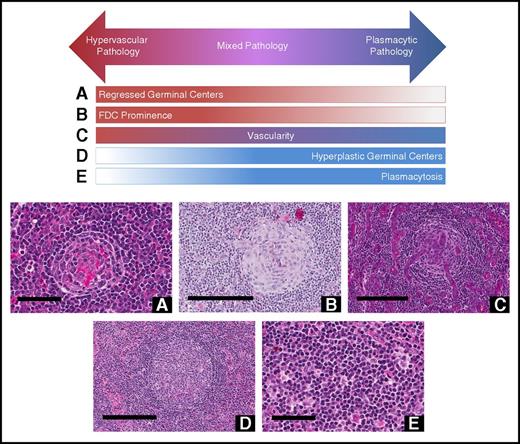
Histopathologic features of CD. Hypervascular subtype is characterized by the presence of regressed germinal centers and FDC prominence, whereas the plasmacytic subtype exhibits hyperplastic germinal centers and profuse plasmacytosis. Mixed subtype exhibits a combination of hypervascular and plasmacytic features. Vascularity is frequently observed in iMCD, but can be seen with either subtype. Deidentified lymph node slides were obtained prestained with hematoxylin and eosin from Janssen Pharmaceuticals and scanned using an Aperio CS scanner (Leica Biosystems, Wetzlar, Germany) at 20×/0.75NA Plan Apochromat. Images were captured using an Aperio Imagescope and enhanced to 300 dpi using Adobe Photoshop. Bars represent 60 μm (A,E), 200 μm (B-D). (A) Regressed germinal center. (B) FDC prominence in germinal center. (C) Blood vessels penetrating germinal center demonstrate prominent vascularity. (D) Hyperplastic germinal center. (E) Sheetlike plasmacytosis.
Histopathologic features of CD. Hypervascular subtype is characterized by the presence of regressed germinal centers and FDC prominence, whereas the plasmacytic subtype exhibits hyperplastic germinal centers and profuse plasmacytosis. Mixed subtype exhibits a combination of hypervascular and plasmacytic features. Vascularity is frequently observed in iMCD, but can be seen with either subtype. Deidentified lymph node slides were obtained prestained with hematoxylin and eosin from Janssen Pharmaceuticals and scanned using an Aperio CS scanner (Leica Biosystems, Wetzlar, Germany) at 20×/0.75NA Plan Apochromat. Images were captured using an Aperio Imagescope and enhanced to 300 dpi using Adobe Photoshop. Bars represent 60 μm (A,E), 200 μm (B-D). (A) Regressed germinal center. (B) FDC prominence in germinal center. (C) Blood vessels penetrating germinal center demonstrate prominent vascularity. (D) Hyperplastic germinal center. (E) Sheetlike plasmacytosis.
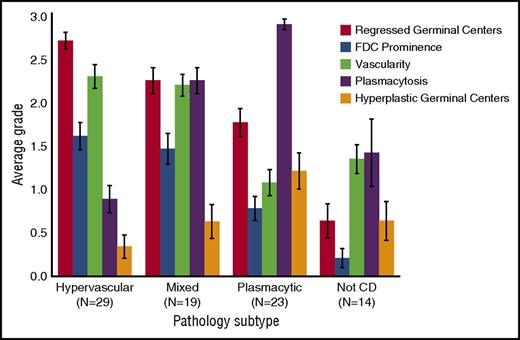
Average grade for histopathologic features for each subtype of iMCD. Average grades for regressed germinal centers, FDC prominence, vascularity, plasmacytosis, and hyperplastic germinal centers in hypervascular, plasmacytic, and mixed subtypes of iMCD as well as cases determined to not be CD (not CD) as assessed by hematopathologic review. See Figure 3 for the grading scale for each feature. The bars depict average grades for histopathologic features for a given subtype with mean ± standard error.
Average grade for histopathologic features for each subtype of iMCD. Average grades for regressed germinal centers, FDC prominence, vascularity, plasmacytosis, and hyperplastic germinal centers in hypervascular, plasmacytic, and mixed subtypes of iMCD as well as cases determined to not be CD (not CD) as assessed by hematopathologic review. See Figure 3 for the grading scale for each feature. The bars depict average grades for histopathologic features for a given subtype with mean ± standard error.
One group of cases (n = 29) showed regressed GCs, FDC prominence, hypervascularization with proliferation of high endothelial venules, and patent sinuses. Mantle zones were also expanded in some cases with “onion skinning,” displayed by concentric rings of small lymphocytes around regressed GCs.9 We sometimes observed the “lollipop sign” of prominent blood vessels radially penetrating GCs and “budding” or “twinning” of follicles, which involves ≥2 GCs located within a single follicle.5,9 Historically, many features of this group would be described as consistent with the “hyaline vascular” (HV) histopathologic subtype of MCD. However, many hematopathologists consider HV to only occur in UCD based on the classic descriptions by Benjamin Castleman, and a few HV-UCD features, such as FDC dysplasia and sclerotic vessels, are not often observed in MCD. Recently, many HV features have been described in iMCD patients with TAFRO syndrome.20 To avoid confusion, we voted to consider iMCD patients with this constellation of HV-like histopathologic features, including regressed GCs and hypervascularization without plasmacytosis, as having the “hypervascular” (HyperV) histopathologic subtype. Of note, most iMCD cases with TAFRO clinical features from our study and the literature demonstrated HyperV or mixed histopathology, but some cases did not.29-31 Also, we observed iMCD patients with HyperV or mixed histopathology that did not have the TAFRO clinical syndrome.
On the other end of the spectrum were patients (n = 23) with sheetlike plasmacytosis and increased numbers of follicles with large hyperplastic GCs.32 These cases, which represent the “plasmacytic” (PC) subtype of iMCD, also have occasional regressed GCs and mild vascularity. A subset of cases (n = 19) demonstrated histologic features that were intermediate between the HyperV and PC subtypes with regressed lymphoid follicles and plasmacytosis, which were considered “mixed.”
The reliability and clinical utility of subtyping into HyperV, PC, and mixed is currently unclear, because there are reports of transitions between variants on subsequent biopsies and simultaneous presence of both subtypes at separate sites within the same patient.33 Nevertheless, histopathologic subtype has been associated with nonresponse to anti-IL-6 therapy.23 Further efforts to validate these histopathologic features in a separate cohort and elucidate the utility of these histopathologic subtypes are needed.
To meet Major Criterion 2, there must be enlarged lymph nodes (≥1 cm in short-axis diameter) in ≥2 lymph node stations. There were inadequate data to support counting splenomegaly toward the minimum of 2 stations. Imaging, such as whole-body computerized tomography, should be performed to assess the number of enlarged lymph node stations. If available,18 [F]-fludeoxyglucose positron emission tomography may help with identifying [F]-fludeoxyglucose-avid nodes for biopsy and distinguishing iMCD from lymphoma. iMCD34 and HHV-8–associated MCD35 may show diffuse lymph node hypermetabolic abnormalities with lower uptake than high-grade lymphomas.36
Minor Criteria
Minor Criteria were selected by the working group from the existing evidence base and subdivided into clinical features and laboratory abnormalities, although additional features may be observed. Clinical Minor Criteria include: constitutional symptoms, hepatosplenomegaly, edema or effusions, eruptive cherry hemangiomatosis or violaceous papules, and lymphocytic interstitial pneumonitis (LIP).23,37 Laboratory Minor Criteria include elevated CRP, anemia, thrombocytopenia or thrombocytosis, hypoalbuminemia, renal dysfunction, and polyclonal hypergammaglobulinemia. CRP should be tracked longitudinally for all patients with iMCD, but erythrocyte sedimentation rate may suffice if CRP is not available. Anemia in iMCD is often microcytic and consistent with anemia of chronic inflammation. Patients often have abnormal platelet counts, with some having thrombocytosis and others having thrombocytopenia. Particularly in TAFRO patients, platelet count tends to reflect iMCD activity, with a drop indicating a flare.20 Experts agreed that there must be at least one laboratory abnormality to diagnose iMCD.
At the conclusion of the meetings, the working group voted to evaluate the minimum number of required Minor Criteria by assessing the number of Minor Criteria at baseline among the 63 patients from NCT01024036 that met the Major Criteria,23 128 cases from the literature review,11 and 25 cases submitted by working group members with patient-level data. The NCT01024036 patients had an average of 3.11 Minor Criteria at the time of enrollment out of the 9 criteria assessed. Data on the presence of LIP and Castleman-specific skin lesions were not systematically captured. Twenty-five cases (40%) met ≥4 Minor Criteria with ≥1 laboratory abnormality, 37 cases (59%) met ≥3 Minor Criteria with ≥1 laboratory abnormality, and 45 cases (71%) met ≥2 Minor Criteria with ≥1 laboratory abnormality at enrollment. The 128 literature review cases had an average of 3.79 Minor Criteria out of an average of 5.11 criteria reported. Sixty cases (47%) met ≥4 Minor Criteria with ≥1 laboratory abnormality, 91 cases (71%) met ≥3 Minor Criteria with ≥1 laboratory abnormality, and 115 cases (90%) met ≥2 Minor Criteria with ≥1 laboratory abnormality. The 10% of cases that did not meet the minimum Minor Criteria had an average of 0.54 reported Minor Criteria. The average number of Minor Criteria was 6.0 out of an average of 10.72 criteria assessed for the 25 cases submitted by working group members with patient-level clinical data. Twenty-two cases (88%) met ≥4 Minor Criteria with ≥1 laboratory abnormality, 24 cases (96%) met ≥3 Minor Criteria with ≥1 laboratory abnormality, and 24 cases (96%) met ≥2 Minor Criteria with ≥1 laboratory abnormality.
Then we evaluated response to therapy according to the NCT01024036 study’s primary end point (decrease in lymph node size as per modified Cheson criteria38 in the absence of symptom progression) for the 54 of 79 patients randomized to the siltuximab arm (Figure 7). Siltuximab-treated patients meeting proposed Major Criteria and ≥2 Minor Criteria with ≥1 laboratory abnormality (n = 35) had a slightly lower overall response rate (43%) than patients with ≥3 Minor Criteria (46%; n = 28) and ≥4 Minor Criteria (55%; n = 22). However, overall response rate dropped significantly (P = .0003) to 0% for the 16 siltuximab-treated patients who did not satisfy our Major Criteria or Minor Criteria threshold. Together, these data supported the minimum threshold of at least 2 Minor Criteria with at least 1 laboratory abnormality for the diagnosis of iMCD.
Percentage of patients meeting proposed Major Criteria who responded to therapy in the siltuximab study, based on number of proposed Minor Criteria. n represents total number of patients treated with at least that number of Minor Criteria and ≥1 laboratory abnormality. Using Fisher’s exact test, those who met ≥2 Minor Criteria were significantly more likely to respond to siltuximab than those who did not meet 2 Minor Criteria (P = .0003).
Percentage of patients meeting proposed Major Criteria who responded to therapy in the siltuximab study, based on number of proposed Minor Criteria. n represents total number of patients treated with at least that number of Minor Criteria and ≥1 laboratory abnormality. Using Fisher’s exact test, those who met ≥2 Minor Criteria were significantly more likely to respond to siltuximab than those who did not meet 2 Minor Criteria (P = .0003).
Exclusion Criteria
The characteristic “Castleman-like” histopathologic changes and clinical abnormalities in iMCD may be present in several malignant, infectious, and autoimmune conditions. For instance, nearly all enlarged lymph nodes from patients with RA and 15% to 30% of SLE display MCD-like histopathology.39-42 Therefore, disorders that can mimic iMCD should be excluded before a diagnosis of iMCD is accepted. The diagnostic evaluation required to exclude other diseases should be based on the clinical presentation, and may require additional biopsies, serologic or microbiology studies as indicated, and careful clinical correlation.
HHV-8–associated MCD can be excluded by negative latency-associated nuclear antigen-1 (LANA-1) staining in a diagnostic lymph node.5 Other virally-associated lymphoproliferations or uncontrolled infections that should be considered include Epstein-Barr virus (EBV)-associated lymphoproliferative disorders, such as infectious mononucleosis or chronic active EBV infection, but low levels of EBV are not necessarily exclusionary.
In addition to SLE and RA, adult-onset Still disease, autoimmune lymphoproliferative syndrome, and juvenile idiopathic arthritis should also be excluded. However, the presence of autoantibodies without a definitive autoimmune diagnosis should not exclude iMCD, because autoantibodies, including anti-nuclear (ANA), anti-platelet, and anti-Sjögren-syndrome–related antigen A (SS-A), or autoimmune hemolytic anemia were found in ∼30% of iMCD patients in the largest series to date.11 A discussion about the overlap between IgG4-related disease (IgG4-RD) and iMCD led to consensus that iMCD should supersede a diagnosis of IgG4-RD, even with very high IgG4 levels, which is in agreement with recommendations from 2 IgG4-RD expert panels.43,44 Dense immunostaining of IgA in the lymph node and low serum IgG4/IgG also support a diagnosis of iMCD over IgG4-RD.45 Hemophagocytic lymphohistiocytosis (HLH) shares significant overlap with iMCD, but our group decided that more data are needed to determine whether HLH should be excluded or considered an associated disease.
The relationship between iMCD and malignancy is poorly understood. iMCD patients have a threefold increased prevalence of malignancy than age-matched controls.11 However, some of those malignancies diagnosed before, concurrently, and shortly after their iMCD diagnosis may have been responsible for the cytokine storm that caused the iMCD-like lymph node histopathology and clinical features. Therefore, we recommend that lymphoma, multiple myeloma, primary lymph node plasmacytoma, and FDC sarcoma should be excluded before diagnosing iMCD. Hematologic malignancies diagnosed more than one year after iMCD with no evidence of the malignancy upon re-review of the diagnostic lymph node or previous imaging should not overturn the initial iMCD diagnosis.
We recommend considering bone marrow biopsy of all patients with suspected iMCD to evaluate for malignancy, POEMS-associated MCD, and findings that can be seen in iMCD.46 POEMS-associated MCD is defined by the presence of either bone lesions (sclerotic or lytic) or a λ-restricted plasma cell disorder as demonstrated by immunofixation, bone marrow aspirate/biopsy, or biopsy of a bone lesion.22 Megakaryocyte hyperplasia and lymphoid aggregates rimmed by plasma cells can be seen in POEMS-associated MCD and iMCD.47 Also, patients meeting the diagnostic criteria for iMCD, who exhibit POEMS-like complications but do not meet the criteria for POEMS, should be considered to have iMCD. More data are needed to better understand the relationship of iMCD to malignancy.
Additional features
Though not included in the Minor Criteria because they lacked sufficient data, additional features that support an iMCD diagnosis include elevated blood levels of IL-6, soluble IL-2 receptor (sIL2R), VEGF, IgA, IgE, lactate dehydrogenase, β-2-microglobulin, characteristic bone marrow pathology (eg, reticulin fibrosis and polyclonal plasmacytosis), and several associated diseases.20,48-53
Discussion
We present the first formal criteria for the diagnosis of iMCD, which require both Major Criteria and at least 2 Minor Criteria including at least 1 laboratory abnormality in the absence of diseases listed in Exclusion Criteria. Taken together, the Major Criteria requirement of characteristic histopathology, clinical and laboratory Minor Criteria, and Exclusion Criteria appear relatively sensitive and specific among causes of multicentric lymphadenopathy. The data regarding response to anti-IL-6 therapy support our threshold of requisite Minor Criteria because all patients who benefited from siltuximab in the clinical trial would have met the threshold.
The role of IL-6 as a mediator of iMCD symptomatology, histopathology, and pathogenesis has been consistently demonstrated.13 Symptoms typically wax and wane with serum IL-6 levels, which are often elevated in iMCD, and many patients respond to IL-6 blockade.54 Although IL-6 was elevated in 57 of 63 patients with iMCD in a recent literature review, IL-6 levels are nonspecific and can be elevated in many inflammatory and malignant disorders.11 Measured IL-6 levels rise after administration of anti-IL-6 therapy, which complicates the interpretation of such data and can falsely suggest that a patient has active iMCD.55 There are also iMCD patients with normal or moderately elevated IL-6 levels and others who do not respond adequately to anti-IL-6 therapy, suggesting that other cytokines may contribute to pathogenesis in these patients. Elevated sIL2R, a marker of T-cell activation, has been found to be frequently elevated in iMCD and may parallel disease activity. VEGF, a potent angiogenic factor, has also been found to be elevated during flares and to rise before other markers of flare, and may be responsible for features such as hypervascularity, cherry hemangiomas, and vascular leak syndrome.11
More research is needed to determine whether patients, who meet Major Criteria and Exclusion Criteria but who do not meet the Minor Criteria, which we consider “probable iMCD,” should be managed in the same way as patients with a definitive diagnosis. From a clinical perspective, physicians are advised to monitor patients closely and/or pursue alternative diagnoses that may explain the multicentric lymphadenopathy in cases with inadequate clinical features. We have also observed patients on the borderline between UCD and iMCD, who have multiple enlarged nodes in one region or in adjacent regions (eg, bilateral cervical or cervical and axillary on one side) and mild clinical symptoms, which we have temporarily referred to as “oligocentric” CD or “regional” CD. More research is needed to determine whether these patients should be treated more like UCD or iMCD. There is also a lack of data regarding optimal management of patients with unresectable UCD. More research is also needed into the differences between iMCD patients with TAFRO syndrome and non-TAFRO/IPL patients, which are both included within these diagnostic criteria.
There are several limitations to our criteria. Though the evidence base was composed of the largest collection of clinical and histologic iMCD data that has ever been analyzed, case reports with short follow-up times make up a portion of cases. We included a broad range of patient data from multiple sources to overcome this limitation, but the actual number of Minor Criteria evaluated from each of the cohorts varied. There was no diagnostic definition for iMCD, so our expert working group had to choose minimum requirements to select cases for the study (“Castleman-like” histopathology, multicentric lymphadenopathy, and negative HHV-8 testing). Additionally, 46 cases from NCT01024036 were previously treated symptomatic patients who did not have clinical data at the time of initial presentation, and the most severe cases were excluded from NCT01024036 and therefore underrepresented. However, NCT01024036 is the only iMCD randomized, controlled trial, and the other 165 cases, which included data at presentation and did not exclude severe cases, had comparable numbers of Minor Criteria. The analysis of response to siltuximab could suggest that this is a predictive response criterion and biased against recognizing cases that will not respond to siltuximab. However, we evaluated response after the criteria were already developed by the working group to evaluate the specificity of the minimum number of Minor Criteria. Also, overlapping diseases were not systematically evaluated in our data set to identify features specific for iMCD. We believe the Exclusion Criteria should help to overcome this limitation until further studies are done to directly compare iMCD against related diseases. These criteria were selected to be applicable to patients worldwide, but violaceous papules and LIP are more commonly described among Asian individuals, and these specific findings were not systematically assessed in NCT01024036.56,57 The expert working group included representation from 8 countries to incorporate patient data from around the world and overcome ascertainment limitations. A portion of the data that informed the working group’s selection of the parameters was later used to evaluate the threshold of required features. Because of the rarity of iMCD and our efforts to collect all available data to assist the expert working group with selecting the parameters, there were no additional data sets to perform separate validation.
In conclusion, the clinical heterogeneity, overlap with other disorders, and lack of specific biomarkers pose challenges for the diagnosis and management of patients with iMCD. Patients are often misdiagnosed with other illnesses and/or forced to endure months without appropriate treatment despite the availability of effective treatments. We believe that these proposed consensus criteria will contribute to streamlining the diagnostic evaluation of patients, standardize nomenclature, and diminish the time from presentation to administration of treatments, which may improve clinical outcomes and survival. The outlined criteria will require collaboration between laboratory physicians and the clinical team. Patients experiencing lymphadenopathy and symptoms listed in the Minor Criteria with no alternative diagnosis should be evaluated for iMCD with an excisional lymph node biopsy.
This international effort represents the first attempt to develop consensus criteria for this rare disease. We recognize the challenges of utilizing retrospective data to define diagnostic criteria, and prospective use of the criteria is necessary for further refinement. We plan to analyze and validate these criteria through the international ACCELERATE Natural History Registry (www.CDCN.org/ACCELERATE), which the CDCN and University of Pennsylvania launched in 2016. All individuals with “Castleman disease” mentioned on a pathology report, including those diagnosed with another disease, can enroll prospectively and central review of clinical data and histopathology are performed. The use of these criteria will facilitate discoveries pertaining to etiology and pathogenesis, assessment of biomarkers, and substratification, which should enable further iterations of these criteria and a precision approach to iMCD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the CDCN and Penn Orphan Disease Center for logistical and funding support and Janssen Pharmaceuticals for providing extensive, deidentified datasets and lymph node slides from clinical trial NCT01024036. All funding from the CDCN and the Penn Orphan Disease Center was provided without further input into the work conduct, results interpretation, and manuscript preparation. The authors also thank Hayley Williamson and Melanie Kier for their assistance with coordinating the meeting and performing preliminary data analyses, Arthur Rubenstein for his guidance, and Noriko Iwaki and Yasuharu Sato for contributing clinical and histopathologic data toward the evidence base for this criteria.
This study was supported, in part, by the Intramural Research Program of the National Institutes of Health.
Authorship
Contribution: D.C.F. and A.Y.L. analyzed and interpreted data, participated in the working group, performed statistical analyses, and wrote the manuscript; S.P. analyzed and interpreted data, performed statistical analyses, and reviewed and approved the final manuscript; and T.S.U., A.B., D.F., D.W., G.S., D.S., D.M., S.C., M.J.L., R.S.M.W., M.P., J.-F.R., M.I., J.R., M.C., A.S., V.K., A.C., G.C., S.N., R.J., C.S.N., C.C., A. Dispenzieri, A.F., D.K., R.K., P.V., A. Dogan, K.Y., F.v.R., E.O., E.S.J., K.S.J.E.-J., and M.S.L. participated in the working group, contributed to the manuscript, and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: D.C.F. and C.C. have received research funding from Janssen Pharmaceuticals and served on advisory boards for Janssen Pharmaceuticals. T.S.U. has a patent for an immunomodulatory compound for KSHV malignancies (Inst). P.V. has served on advisory boards for Janssen Pharmaceuticals. D.S. has received research funding from Amgen and honoraria from Celgene, Roche, and Janssen Pharmaceuticals. R.S.M.W. has performed consultancy for Novartis, Bayer, Boehringer-Ingelheim, and GlaxoSmithKline and received research funding from Alexion, Baxalta, Bayer, Biogen-Idec, Boehringer-Ingelheim, GlaxoSmithKline, Janssen, MSD, Novartis, Omeros, Pfizer, and Sanofi. R.K. has received research funding from Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant, as well as consultant/advisory board fees from Actuate Therapeutics and Xbiotech, and an ownership interest in Novena, Inc. and Curematch, Inc. F.v.R. has performed consultancy for Takeda and Amgen. A.F. has received honoraria from Janssen Pharmaceuticals. A. Dogan has served on an advisory board for Cancer Genetics and been a speaker for the Peer Review Institute. The remaining authors declare no competing financial interests.
Correspondence: David C. Fajgenbaum, Hospital of the University of Pennsylvania, 3400 Spruce St, Silverstein, Suite S05094, Philadelphia, PA 19104; e-mail: davidfa@mail.med.upenn.edu; Elaine S. Jaffe, Center for Cancer Research, National Cancer Institute, Building 10, Room 3S235, Bethesda, MD 20892-1500; e-mail: ejaffe@mail.nih.gov; Thomas S. Uldrick, Center for Cancer Research, National Cancer Institute, Building 10, Room 6N106, Bethesda, MD 20892; e-mail: uldrickts@mail.nih.gov; Kojo S. J. Elenitoba-Johnson, Department of Pathology and Laboratory Medicine, Perelman School of Medicine at the University of Pennsylvania, 609 Stellar-Chance, Philadelphia, PA 19104-6100; e-mail: kojo.elenitoba-johnson@uphs.upenn.edu; Megan S. Lim, Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, 609 Stellar-Chance, 422 Curie Blvd, Philadelphia, PA 19104; e-mail: megan.lim@uphs.upenn.edu.