Key Points
Clofarabine integrated in standard induction therapy for newly diagnosed AML reduces relapse probability but does not improve survival.
Clofarabine improves survival in intermediate-risk AML categories ELN-1 and the AML genotype without NPM1 and without FLT3-ITD gene mutations.
Abstract
Clofarabine has demonstrated antileukemic activity in acute myeloid leukemia (AML) but has yet to be critically evaluated in younger adults in the frontline with standard chemotherapy. We compared 2 induction regimens in newly diagnosed patients ages 18 to 65 with acute myeloid leukemia (AML)/high-risk myelodysplastic syndromes, that is, idarubicine-cytarabine (cycle I) and amsacrine-cytarabine (cycle II) without or with clofarabine (10 mg/m2 on days 1-5 of each of both cycles). Consolidation involved chemotherapy with or without hematopoietic stem cell transplantation. Event-free survival (EFS, primary endpoint) and other clinical endpoints and toxicities were assessed. We randomized 402 and 393 evaluable patients to the control or clofarabine induction treatment arms. Complete remission rates (89%) did not differ but were attained faster with clofarabine (66% vs 75% after cycle I). Clofarabine added grades 3 to 4 toxicities and delayed hematological recovery. At a median follow-up of 36 months, the study reveals no differences in overall survival and EFS between the control (EFS, 35% ± 3 [standard error] at 4 years) and clofarabine treatments (38% ± 3) but a markedly reduced relapse rate (44% ± 3 vs 35% ± 3) in favor of clofarabine and an increased death probability in remission (15% ± 2 vs 22% ± 3). In the subgroup analyses, clofarabine improved overall survival and EFS for European Leukemia Net (ELN) 2010 intermediate I prognostic risk AML (EFS, 26% ± 4 vs 40% ± 5 at 4 years; Cox P = .002) and for the intermediate risk genotype NPM1 wild-type/FLT3 without internal-tandem duplications (EFS, 18% ± 5 vs 40% ± 7; Cox P < .001). Clofarabine improves survival in subsets of intermediate-risk AML only. HOVON-102 study is registered at Netherlands Trial Registry #NTR2187.
Introduction
With current remission induction chemotherapy that usually includes cytarabine (Ara-C) and one of the anthracyclines, high rates of complete remissions can be obtained in newly diagnosed patients with acute myeloid leukemia (AML) of young and middle age. However, a high frequency of subsequent relapse remains the major hurdle to clear. In adults younger than 65 years of age the average probability of relapse is above 50% even though various postremission therapeutic strategies, including stem cell transplantation approaches, are being applied. The addition of new drugs to the classical “7+3”-based remission induction schedule of 3 days of daunorubicin or idarubicin plus 7 days of cytarabine has not resulted in an overall significant therapeutic survival advance.
Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-d-arabinofuranosyladenine) is a second-generation nucleoside analog, and it is an active therapeutic agent in AML. Monotherapy with the drug has shown substantial response rates in relapsing or refractory patients who had failed prior therapy1 as well as in newly diagnosed patients of older age who are considered unsuitable for intensive chemotherapy.2 In combination with cytarabine-based regimens in relapsed and refractory patients with AML, clofarabine has demonstrated acceptable associated toxicity.3 A large phase III trial, in which clofarabine (at 40 mg/m2) has been combined with cytarabine at an intermediate dose of 1000 mg/m2 for 5 days in comparison with cytarabine alone, demonstrated more complete responses (CRs) and a better event-free survival (EFS), but overall survival (OS) was not improved.4 The latter study was restricted to patients of older age (in this case, older than 55 years old) and patients with relapsed/refractory AML. These studies in older patients or patients with relapsed/refractory AML were done with relatively high-dose levels of clofarabine (20-40 mg/m2), and although they showed promising response rates,2,4 they also showed more toxicity. Only one study has been published that has evaluated clofarabine in younger adults with AML in a combination with both an anthracyclin and cytarabine.5 This study with clofarabine at 20 mg/m2 suggested an enhanced survival for the clofarabine combination in relation to historical controls, but it was a single-arm study in 59 patients only. Thus, a more robust critical prospective assessment of the therapeutic value of clofarabine integrated in the upfront setting of intensive chemotherapy and especially in younger and middle-aged adults has been lacking.
Here we report such a phase III study of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON)–Swiss Group for Clinical Cancer Research (SAKK) Cooperative Groups in newly diagnosed patients ages 18 to 65 years old with AML or high-risk myelodysplastic syndromes (MDS) (refractory anemia with excess blasts [RAEB] with International Prognostic Scoring Scale [IPSS] ≥ 1.5) in which a backbone of 2 successive cycles of standard induction chemotherapy was prospectively compared with a similar regimen to which clofarabine was added. Thus we set out to prospectively evaluate the efficacy and safety of a remission induction program with clofarabine as an adjunct to induction cycle I and cycle II.
Methods and patients
Eligibility
Previously untreated adults who were 18 to 65 years of age with a cytopathologically confirmed diagnosis of AML, or with RAEB and an international prognostic score of ≥1.5 IPSS,6 a World Health Organization (WHO) performance status of ≤2, and a written informed consent, were eligible. Exclusion criteria are specified in the supplemental Appendix, available on the Blood Web site.
Study design and chemotherapy
Patients were randomly assigned to remission induction regimens with or without clofarabine. Cycle I of the control arm included idarubicin at 12 mg/m2 (3-hour infusion on days 1, 2, and 3) and cytarabine at a dose of 200 mg/m2 (per continuous infusion on days 1-7) with or without clofarabine at 10 mg/m2 per 1 hour of infusion on days 1 to 5. Cycle II contained amsacrine 120 mg/m2 per 1-hour infusion on days 4, 5, and 6 plus cytarabine 1000 mg/m2 given intravenously for 3 hours twice daily on days 1-67 with or without the addition of clofarabine 10 mg/m2 given intravenously for 1 hour on days 1 to 5.
Patients in complete remission (CR or CRi) after cycle II were assigned to consolidation with additional chemotherapy with mitoxantrone-etoposide (cycle III), or autologous hematopoietic stem cell transplantation following busulfan-cyclophosphamide or total body irradiation-cyclophosphamide pretreatment or allogeneic stem cell transplantation (alloSCT) depending on their prognostic risk status as described8-10 (detailed in the supplemental Appendix). The study began with a dose-selection run-in phase with dose levels of 15 mg/m2 and 10 mg/m2 of clofarabine, and after evaluation of the toxicity profiles, the study was continued with clofarabine at 10 mg/m2 as an open-label phase III trial (see the supplemental Appendix).
The study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All patients gave their written informed consent.
Risk classification and clinical characteristics
On the basis of the karyotype and molecular genotype of the leukemic cells, patients were classified into prognostic categories. Patients with prior MDS or prior hematological disease (including myeloproliferative diseases) are classified as secondary AML (sAML). Patients with prior chemotherapy or radiotherapy (and no secondary AML) are classified as therapy- related AML (tAML). WHO performance status, extramedullary disease, and white blood cell count (WBC) were registered at diagnosis.
Criteria for response and endpoints
Criteria for complete response (CR) and complete response with insufficient hematological recovery (CRi) and relapse were previously defined.11 EFS refers to the interval from randomization to the date of failure to enter a complete remission within 2 cycles, death, or relapse, whichever occurs first. OS was measured from randomization. Relapse-free survival (RFS) was defined for all patients who attained CR on protocol and measured from the date of CR until relapse or death in CR.
Molecular analyses
Targeted sequencing and other methods used for the molecular assessment of common AML-associated gene mutations and high-EVI1 mRNA expression on bone marrow or blood specimens at diagnosis are described in the supplemental Appendix, and the molecular biomarkers are listed in Table 1.
Patient characteristics at baseline according randomization
| . | Control induction treatment . | Clofarabine induction treatment . | No. of cases evaluated . |
|---|---|---|---|
| Total | 402 (100%) | 393 (100%) | |
| Sex, male | 215 (53%) | 229 (58%) | |
| Age, y | |||
| Median (range)* | 54 (18-65) | 56 (18-66) | |
| ≤45 | 108 (27%) | 112 (28%) | |
| 46-60 | 195 (49%) | 166 (42%) | |
| 61-65 | 99 (25%) | 115 (29%) | |
| Performance status | |||
| WHO 0 | 206 (51%) | 178 (45%) | |
| WHO 1 | 152 (38%) | 170 (43%) | |
| WHO 2 | 29 (7%) | 28 (7%) | |
| Unknown | 15 (4%) | 17 (4%) | |
| AML type | |||
| De novo | 346 (86%) | 341 (87%) | |
| sAML | 35 (9%) | 33 (8%) | |
| tAML | 21 (5%) | 19 (5%) | |
| High-risk RAEB | 42 (10%) | 38 (10%) | |
| First-degree relatives with AML | 9 (2%) | 7 (2%) | |
| Family history unknown | 51 (13%) | 49 (12%) | |
| WBC at diagnosis [×109/L] | |||
| ≤20 | 282 (70%) | 281 (72%) | |
| 20-100 | 94 (23%) | 87 (22%) | |
| >100 | 25 (6%) | 24 (6%) | |
| Median (range) | 6.4 (0.4-341) | 7.3 (0.2-229) | |
| Blasts (%) in bone marrow (median) | 51 | 51 | |
| Cytogenetics | |||
| t(8;21)* | 24 (6%) | 21 (5%) | |
| inv(16) | 14 (3%) | 15 (4%) | |
| CN X-Y | 208 (52%) | 184 (47%) | |
| CA rest | 94 (23%) | 105 (27%) | |
| Monosomal karyotype | 53 (13%) | 52 (13%) | |
| Unknown | 9 (2%) | 16 (4%) | |
| Gene mutations | |||
| NPM1 | 116 (31%) | 95 (25%) | 754 |
| FLT3-ITD | 68 (19%) | 71 (20%) | 704 |
| FLT3-TKD835 | 24 (7%) | 24 (7%) | 653 |
| NPM1 mut-FLT3-ITD neg | 69 (20%) | 50 (14%) | 696 |
| NPM1 mut-FLT3-ITD | 35 (10%) | 39 (11%) | |
| NPM1wt-FLT3-ITD neg | 209 (60%) | 229 (65%) | |
| NPM1wt-FLT3-ITD | 33 (10%) | 32 (9%) | |
| DNMT3A mutation | 82 (27%) | 85 (27%) | 609 |
| IDH1 mutation | 28 (9%) | 34 (11%) | 609 |
| IDH2 mutation | 34 (11%) | 36 (12%) | 609 |
| TET2 mutation | 34 (11%) | 41 (13%) | 609 |
| Bi-allelic CEBPA mutations | 12 (4%) | 14 (5%) | 573 |
| RUNX1 mutation | 40 (13%) | 40 (13%) | 609 |
| ASXL1 mutation | 31 (10%) | 32 (10%) | 609 |
| P53 mutation | 28 (9%) | 29 (9%) | 609 |
| SF3B1 mutation | 9 (3%) | 4 (1%) | 609 |
| SRSF2 mutation | 24 (8%) | 31 (10%) | 609 |
| PTPN11 mutation | 33 (11%) | 36 (12%) | 609 |
| KRAS mutation | 19 (6%) | 21 (7%) | 609 |
| NRAS mutation | 59 (20%) | 70 (23%) | 609 |
| MLL-PTD | 16 (7%) | 13 (5%) | 490 |
| JAK2 | 7 (2%) | 8 (3%) | 609 |
| EVI-1 overexpression | 36 (11%) | 46 (14%) | 641 |
| Prognostic risk according to ELN criteria 2010† | |||
| Favorable | 104 (26%) | 85 (22%) | |
| Intermediate I | 121 (30%) | 123 (31%) | |
| Intermediate II | 88 (22%) | 99 (25%) | |
| Adverse | 89 (22%) | 86 (22%) | |
| . | Control induction treatment . | Clofarabine induction treatment . | No. of cases evaluated . |
|---|---|---|---|
| Total | 402 (100%) | 393 (100%) | |
| Sex, male | 215 (53%) | 229 (58%) | |
| Age, y | |||
| Median (range)* | 54 (18-65) | 56 (18-66) | |
| ≤45 | 108 (27%) | 112 (28%) | |
| 46-60 | 195 (49%) | 166 (42%) | |
| 61-65 | 99 (25%) | 115 (29%) | |
| Performance status | |||
| WHO 0 | 206 (51%) | 178 (45%) | |
| WHO 1 | 152 (38%) | 170 (43%) | |
| WHO 2 | 29 (7%) | 28 (7%) | |
| Unknown | 15 (4%) | 17 (4%) | |
| AML type | |||
| De novo | 346 (86%) | 341 (87%) | |
| sAML | 35 (9%) | 33 (8%) | |
| tAML | 21 (5%) | 19 (5%) | |
| High-risk RAEB | 42 (10%) | 38 (10%) | |
| First-degree relatives with AML | 9 (2%) | 7 (2%) | |
| Family history unknown | 51 (13%) | 49 (12%) | |
| WBC at diagnosis [×109/L] | |||
| ≤20 | 282 (70%) | 281 (72%) | |
| 20-100 | 94 (23%) | 87 (22%) | |
| >100 | 25 (6%) | 24 (6%) | |
| Median (range) | 6.4 (0.4-341) | 7.3 (0.2-229) | |
| Blasts (%) in bone marrow (median) | 51 | 51 | |
| Cytogenetics | |||
| t(8;21)* | 24 (6%) | 21 (5%) | |
| inv(16) | 14 (3%) | 15 (4%) | |
| CN X-Y | 208 (52%) | 184 (47%) | |
| CA rest | 94 (23%) | 105 (27%) | |
| Monosomal karyotype | 53 (13%) | 52 (13%) | |
| Unknown | 9 (2%) | 16 (4%) | |
| Gene mutations | |||
| NPM1 | 116 (31%) | 95 (25%) | 754 |
| FLT3-ITD | 68 (19%) | 71 (20%) | 704 |
| FLT3-TKD835 | 24 (7%) | 24 (7%) | 653 |
| NPM1 mut-FLT3-ITD neg | 69 (20%) | 50 (14%) | 696 |
| NPM1 mut-FLT3-ITD | 35 (10%) | 39 (11%) | |
| NPM1wt-FLT3-ITD neg | 209 (60%) | 229 (65%) | |
| NPM1wt-FLT3-ITD | 33 (10%) | 32 (9%) | |
| DNMT3A mutation | 82 (27%) | 85 (27%) | 609 |
| IDH1 mutation | 28 (9%) | 34 (11%) | 609 |
| IDH2 mutation | 34 (11%) | 36 (12%) | 609 |
| TET2 mutation | 34 (11%) | 41 (13%) | 609 |
| Bi-allelic CEBPA mutations | 12 (4%) | 14 (5%) | 573 |
| RUNX1 mutation | 40 (13%) | 40 (13%) | 609 |
| ASXL1 mutation | 31 (10%) | 32 (10%) | 609 |
| P53 mutation | 28 (9%) | 29 (9%) | 609 |
| SF3B1 mutation | 9 (3%) | 4 (1%) | 609 |
| SRSF2 mutation | 24 (8%) | 31 (10%) | 609 |
| PTPN11 mutation | 33 (11%) | 36 (12%) | 609 |
| KRAS mutation | 19 (6%) | 21 (7%) | 609 |
| NRAS mutation | 59 (20%) | 70 (23%) | 609 |
| MLL-PTD | 16 (7%) | 13 (5%) | 490 |
| JAK2 | 7 (2%) | 8 (3%) | 609 |
| EVI-1 overexpression | 36 (11%) | 46 (14%) | 641 |
| Prognostic risk according to ELN criteria 2010† | |||
| Favorable | 104 (26%) | 85 (22%) | |
| Intermediate I | 121 (30%) | 123 (31%) | |
| Intermediate II | 88 (22%) | 99 (25%) | |
| Adverse | 89 (22%) | 86 (22%) | |
N refers to number of patients. Gene mutations are as follows: NPM1 nuclephosmin-1; FLT3, fms-like tyrosine kinase-3; FLT3-TKD835, FLT3 gene with point mutation at position D835; DNMT3A, DNA methyltransferase 3A; IDH1/IDH2, isocitrate dehydrogenase 1 and 2; TET2, Ten-Eleven translocation-2; CEPBA, CCAAT/enhancer-binding protein α; RUNX1, runt-related transcription factor 1; ASXL1, additional sex combs like 1; p53; SF3B1, splicing factor 3B subunit; SRSF2, serine and arginine rich splicing factor 2; PTPN11, protein tyrosine phosphatase, nonreceptor type 11; MLL, myeloid/lymphoid or mixed-lineage leukemia; JAK2, Janus kinase 2. RAEB with an international prognostic score of ≥1.5 IPSS is as described previously.6 The ELN prognostic risk categories are as described by Döhner et al.12
CA, abnormal cytogenetics; CN, normal cytogenetics; EVI1, ecotropic virus integration 1 gene; FLT3-ITD negative, FLT3 without internal tandem duplications; ITD, internal tandem duplication; mut, mutation; neg, negative; PTD, partial tandem duplication; sAML, secondary AML (after myelodysplastic syndrome and antecedent hematological disease); tAML, therapy-related AML (in case of prior chemotherapy or radiotherapy. For details, see the Methods section); WBC, white blood cell count at diagnosis; WHO, World Health Organization.
AML with core-binding factor abnormalities: t(8;21) (q22;q22), inv(16)(p13.1;q22), or t(16;16)(p13.1;q22) Monosomal karyotype is defined as described by Breems et al.13
According to Döhner et al12 but slightly modified for bi-allelic CEBPA gene mutations, as detailed in the supplemental Appendix.
Statistical analysis
Randomizations were balanced with a biased-coin minimization procedure, with the bias dependent on the average imbalance between the numbers of patients already assigned to each group overall, within the participating hospital, and within the diagnostic subgroups (AML or RAEB) of the newly diagnosed patient. This randomized, open-label, phase III study has been designed to study the therapeutic value of clofarabine as an adjunct to standard remission-induction chemotherapy in previously untreated AML or high-risk MDS (RAEB, IPSS ≥ 1.5), ages 18 to 65 years old. The study started with a dose-finding phase, after which the study was continued as phase III.
The first patient was registered in the study on February 25, 2010, and the study was closed when the target number of patients had been reached. The last patient was registered on September 28, 2013.
This report presents the results of the final analysis of the phase III part of the study. The primary endpoint of the study is EFS, and the study is powered on this endpoint. The target number of 800 patients, to be accrued in 4 years with an additional follow-up of 1 year after registration of the last patient, would result in 509 events and give a power of 87% with a 2-sided test at a 5% significance level to detect an improvement of EFS with a hazard ratio (HR) of 0.76. This improvement corresponds to an increase of EFS at 3 years from 31% to 41%. All analyses other than those related to the primary endpoint can be considered exploratory.
When more than 2 years after the last patient had been enrolled, the number of 509 events for EFS had still not been reached, the Data Safety Monitoring Committee recommended to wait no longer for additional events and to perform the final study analysis.
All analyses were done according to the intention-to-treat principle irrespective of protocol compliance. Ten (control arm) and 20 (clofarabine arm) registered patients turned out to be ineligible afterward and were excluded from all analyses (specified in the supplemental Appendix). Cox regression analysis was used to analyze the effect of treatment on EFS, OS, and RFS with and without adjustment for other covariates. Subgroup analyses were done for a limited number of subgroups: by age (3 groups of similar size), WHO performance status at entry, type of leukemia (sAML, tAML, de novo), AML versus RAEB, and European Leukemia Net (ELN) risk 2010.12 The power of these subgroup analyses was limited, because the trial was not designed for that. Competing risk analysis was applied with regard to type of failures in EFS and RFS.
Hematological recovery after induction cycles I and II and consolidation cycle III was analyzed actuarially from the first day of chemotherapy and compared between the groups with the log-rank test. In these analyses, patients were censored for hematological recovery at death or at start of next treatment if they had not yet recovered at that time point. All P values are 2 sided and not adjusted for multiple testing.
Results
Patients
During the initial dose-finding run-in phase of the study (August-November 2010), 54 patients at a dose level of 10 mg/m2 clofarabine and 65 patients at a dose level of 15 mg/m2 were prospectively randomized to assess feasibility. Because of an increased infection rate and other dose-limiting toxicities (7 vs 2) noted among the clofarabine 15 mg/m2 treatment group, the dose of clofarabine at 10 mg/m2 was selected for the phase III study (supplemental Appendix). Until September 28, 2013, 795 eligible and evaluable patients with AML (n = 715) or RAEB (n = 80) were randomized. Four hundred and two patients were assigned to the standard induction regimen and 393 to the clofarabine induction regimen (for Consort diagram see supplemental Figure 1). The median follow-up of patients still alive at the date of last contact (n = 381) is 36 months. Table 1 presents the demographic characteristics of the patients. Median age was 55 years, with 27% of patients between 61 and 66 years of age. The 2 groups were comparable regarding clinical, hematological, and cytogenetic features.
Treatment, response, and outcome
Of 795 eligible patients, 769 (97%) received induction cycle I at full dose according to schedule, and 634 of 688 (92%) received induction cycle II at full dose according to schedule, with similar distributions between both treatment arms (for Consort Diagram see supplemental Figure 1; Table 2). Patients assigned to the control arm and clofarabine treatment arm showed similar CR rates on induction (85% vs 84%) and CRi rates (4% vs 5%), but earlier CRs were attained on the clofarabine treatment arm (ie, 66% vs 75% after the first cycle I) (Table 2). Among complete responders who had completed cycles I and II, 206 (26%) received chemotherapy cycle III for consolidation, and 66 (8%) proceeded to an autologous hematopoietic stem cell transplantation and 331 (42%) patients to alloSCT (allogeneic stem cell transplantation), with no differences between the treatment groups (Table 2).
Treatment and outcomes
| . | . | . | Logistic/Cox regression . | ||||
|---|---|---|---|---|---|---|---|
| . | Control induction therapy . | Clofarabine induction therapy . | OR/HR . | 95% CI . | P . | ||
| Total | 402 (100%) | 393 (100%) | |||||
| Treatments | |||||||
| Remission induction | |||||||
| Cycle I | 401 (100%) | 393 (100%) | |||||
| Cycle II | 360 (90%) | 328 (83%) | |||||
| Consolidation after CR | 301 (75%) | 244 (61%) | |||||
| Consolidation therapy | |||||||
| Cycle III | 97 (24%) | 51 (13%) | |||||
| Autologous SCT | 35 (9%) | 31 (8%) | |||||
| Allogeneic SCT | 169 (42%) | 162 (41%) | |||||
| Outcomes | |||||||
| Complete remission (CR/CRi) | 355 (88%) | 352 (90%) | 1.14 | 0.73-1.77 | .57 | ||
| Early CR/CRi (after cycle I) | 267 (66%) | 293 (75%) | |||||
| Late CR/CRi | 88 (22%) | 59 (15%) | |||||
| Early death | |||||||
| Early death (<30 days) | 18 (4%) | 21 (5%) | |||||
| Death within 60 days | 32 (8%) | 33 (8%) | |||||
| Event-free survival (EFS) at 4 years (actuarial survival, % ± SE) | |||||||
| EFS | 35% (SE = 3%) | 38% (SE = 3%) | 0.90 | 0.75-1.07 | .24 | ||
| No CR | 15% (SE = 2%) | 13% (SE = 2%) | |||||
| Relapse | 38% ± 3 | 30% ± 2 | |||||
| Death | 13% ± 2 | 19% ± 2 | |||||
| Overall survival (OS) at 4 years (numbers, actuarial survival, % ± SE) | |||||||
| OS at 4 years | 43% ± 3 | 44% ± 3 | 0.95 | 0.78-1.15 | .57 | ||
| Relapse free at 4 years (actuarial, % ± SE) | |||||||
| RFS | 41% ± 3 | 44% ± 3 | 0.90 | 0.74-1.10 | .32 | ||
| Relapse | 44% ± 3 | 35% ± 3 | |||||
| Death | 15% ± 2 | 22% ± 3 | |||||
| . | . | . | Logistic/Cox regression . | ||||
|---|---|---|---|---|---|---|---|
| . | Control induction therapy . | Clofarabine induction therapy . | OR/HR . | 95% CI . | P . | ||
| Total | 402 (100%) | 393 (100%) | |||||
| Treatments | |||||||
| Remission induction | |||||||
| Cycle I | 401 (100%) | 393 (100%) | |||||
| Cycle II | 360 (90%) | 328 (83%) | |||||
| Consolidation after CR | 301 (75%) | 244 (61%) | |||||
| Consolidation therapy | |||||||
| Cycle III | 97 (24%) | 51 (13%) | |||||
| Autologous SCT | 35 (9%) | 31 (8%) | |||||
| Allogeneic SCT | 169 (42%) | 162 (41%) | |||||
| Outcomes | |||||||
| Complete remission (CR/CRi) | 355 (88%) | 352 (90%) | 1.14 | 0.73-1.77 | .57 | ||
| Early CR/CRi (after cycle I) | 267 (66%) | 293 (75%) | |||||
| Late CR/CRi | 88 (22%) | 59 (15%) | |||||
| Early death | |||||||
| Early death (<30 days) | 18 (4%) | 21 (5%) | |||||
| Death within 60 days | 32 (8%) | 33 (8%) | |||||
| Event-free survival (EFS) at 4 years (actuarial survival, % ± SE) | |||||||
| EFS | 35% (SE = 3%) | 38% (SE = 3%) | 0.90 | 0.75-1.07 | .24 | ||
| No CR | 15% (SE = 2%) | 13% (SE = 2%) | |||||
| Relapse | 38% ± 3 | 30% ± 2 | |||||
| Death | 13% ± 2 | 19% ± 2 | |||||
| Overall survival (OS) at 4 years (numbers, actuarial survival, % ± SE) | |||||||
| OS at 4 years | 43% ± 3 | 44% ± 3 | 0.95 | 0.78-1.15 | .57 | ||
| Relapse free at 4 years (actuarial, % ± SE) | |||||||
| RFS | 41% ± 3 | 44% ± 3 | 0.90 | 0.74-1.10 | .32 | ||
| Relapse | 44% ± 3 | 35% ± 3 | |||||
| Death | 15% ± 2 | 22% ± 3 | |||||
CI, confidence interval; CR, complete remission on protocol; early CR, complete remission attained after remission induction cycle I; HR, hazard ratio; late CR, complete remission attained after cycle II; OR, odds ratio; RFS, relapse free survival; SCT, hematopoietic stem cell transplantation; SE, standard error.
In the control arm, 148 patients relapsed, and 216 have died, including 48 in the first CR. Among the clofarabine treatment group, 114 patients relapsed and 198 have died, including 66 in the first CR.
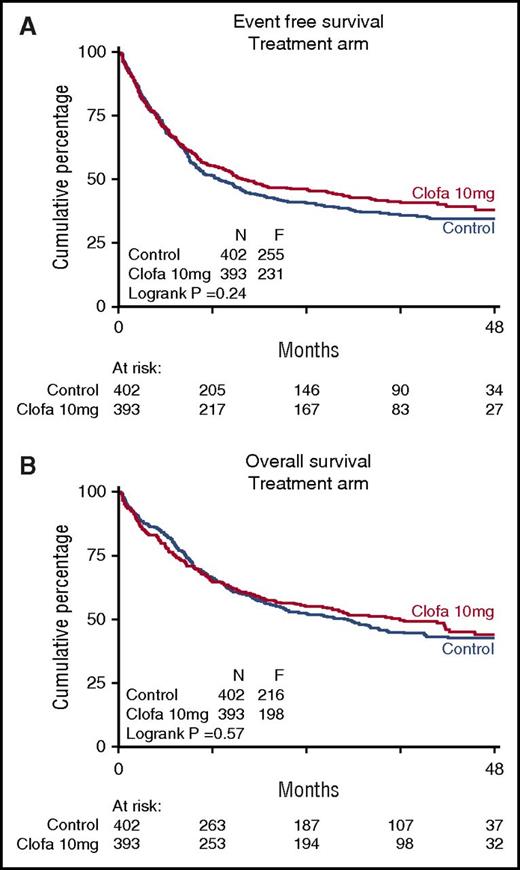
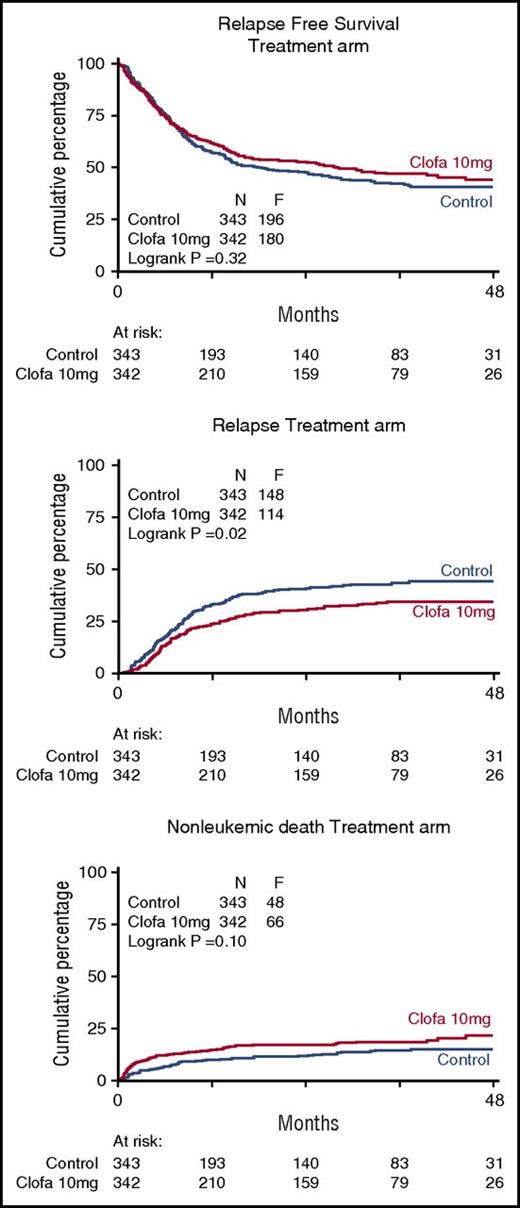
There were no apparent differences between the 2 study groups in EFS at 4 years (EFS 35% ± 3 vs 38% ± 3; Cox P = .24), or OS (43% ± 3 vs 44% ± 3; Cox P = .57) (Table 3; Figure 1) nor with regard to RFS for the complete responders (41% ± 3 vs 44% ± 3 at 4 years; Cox P = .32). The relapse rate among patients attaining a CR/CRi (as competing risk in RFS) was better in the clofarabine treatment group (Table 2; Figure 2) with 4-year estimates of 44% ± 3 (control group) versus 35% ± 3 (clofarabine treatment arm). On the other hand, the cumulative 4-year probabilities for the competing risk of death in first CR/CRi were greater in the clofarabine group (death in CR 15% ± 2 vs 22% ± 3) (Table 2; Figure 2). The latter results are indicative of a greater antileukemic effect of the clofarabine schedule and of a concurrent greater toxicity profile, resulting in an enhanced death rate in CR.
Comparative effect of remission induction therapy without (control) and with additional clofarabine on overall survival and event-free survival in relationship to patient age, white blood cell count at diagnosis, WHO performance status, secondary AML/therapy-related AML, and ELN prognostic category
| . | . | Overall survival at 4 years . | Event-free survival at 4 years . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | Treatment group . | . | Treatment group . | . | |||
| . | N . | Control (%) . | Clofarabine (%) . | P . | Control (%) . | Clofarabine (%) . | P . | |
| Total | 795 | 43% | 44% | .57 | 35% | 38% | .24 | |
| Age, y | ||||||||
| ≤45 | 220 | 60% | 61% | .59 | 47% | 54% | .22 | |
| 46-60 | 361 | 45% | 46% | 1.0 | 37% | 38% | .84 | |
| 61-65 | 214 | 20% | 26% | .47 | 17% | 24% | .38 | |
| WHO performance status | ||||||||
| WHO 0 | 384 | 40% | 52% | .04 | 36% | 44% | .04 | |
| WHO >0 | 411 | 45% | 38% | .30 | 33% | 33% | .80 | |
| Type of AML | ||||||||
| De novo | 687 | 44% | 46% | .43 | 35% | 41% | .10 | |
| sAML | 68 | 28% | 18% | .48 | 25% | 8% | .36 | |
| tAML | 40 | 42% | 47% | .99 | 39% | 37% | .66 | |
| ELN 2010 risk | ||||||||
| Favorable | 189 | 70% | 66% | .37 | 55% | 64% | .74 | |
| Intermediate I | 244 | 29% | 50% | <.001 | 26% | 40% | .002 | |
| Intermediate II | 187 | 54% | 44% | .40 | 41% | 37% | .75 | |
| Adverse | 175 | 15% | 16% | .49 | 16% | 13% | .91 | |
| Composite FLT3-ITD/NPM1 genotype | ||||||||
| NPM1wt/FLT3-ITD neg | 135 | 22% | 49% | <.001 | 18% | 40% | <.001 | |
| . | . | Overall survival at 4 years . | Event-free survival at 4 years . | |||||
|---|---|---|---|---|---|---|---|---|
| . | . | Treatment group . | . | Treatment group . | . | |||
| . | N . | Control (%) . | Clofarabine (%) . | P . | Control (%) . | Clofarabine (%) . | P . | |
| Total | 795 | 43% | 44% | .57 | 35% | 38% | .24 | |
| Age, y | ||||||||
| ≤45 | 220 | 60% | 61% | .59 | 47% | 54% | .22 | |
| 46-60 | 361 | 45% | 46% | 1.0 | 37% | 38% | .84 | |
| 61-65 | 214 | 20% | 26% | .47 | 17% | 24% | .38 | |
| WHO performance status | ||||||||
| WHO 0 | 384 | 40% | 52% | .04 | 36% | 44% | .04 | |
| WHO >0 | 411 | 45% | 38% | .30 | 33% | 33% | .80 | |
| Type of AML | ||||||||
| De novo | 687 | 44% | 46% | .43 | 35% | 41% | .10 | |
| sAML | 68 | 28% | 18% | .48 | 25% | 8% | .36 | |
| tAML | 40 | 42% | 47% | .99 | 39% | 37% | .66 | |
| ELN 2010 risk | ||||||||
| Favorable | 189 | 70% | 66% | .37 | 55% | 64% | .74 | |
| Intermediate I | 244 | 29% | 50% | <.001 | 26% | 40% | .002 | |
| Intermediate II | 187 | 54% | 44% | .40 | 41% | 37% | .75 | |
| Adverse | 175 | 15% | 16% | .49 | 16% | 13% | .91 | |
| Composite FLT3-ITD/NPM1 genotype | ||||||||
| NPM1wt/FLT3-ITD neg | 135 | 22% | 49% | <.001 | 18% | 40% | <.001 | |
Actuarial estimates and log-rank test P values for difference between both treatment groups within subgroups are given. ELN risk 2010 according to Döhner et al12 was slightly modified for bi-allelic CEBPA gene mutations, as detailed in the supplemental Appendix. NPM1 wild-type refers to nonmutated nuclephosmin-1 gene; FLT3-ITD negative refers to fms-like tyrosine kinase-3 with no internal tandem duplications. The composite NPM1wt/FLT3-ITD neg is of intermediate prognostic risk. ELN 2010 prognostic risk categories are described by Döhner et al.12 Bold values indicate significant statistical differences.
N, number of patients.
Treatment arms. Event-free survival (A) and overall survival (B) of patients on remission induction therapy (control group) versus clofarabine therapy group are shown. Patients were randomized for their first (I) and second (II) induction cycles of combination chemotherapy without additional clofarabine (control arm) or with clofarabine at 10 mg/m2 on days 1 to 5 of each of both cycles (clofarabine treatment). Clofa, clofarabine.
Treatment arms. Event-free survival (A) and overall survival (B) of patients on remission induction therapy (control group) versus clofarabine therapy group are shown. Patients were randomized for their first (I) and second (II) induction cycles of combination chemotherapy without additional clofarabine (control arm) or with clofarabine at 10 mg/m2 on days 1 to 5 of each of both cycles (clofarabine treatment). Clofa, clofarabine.
Relapse-free survival of complete responders and competing risk of relapse and death in complete remission (CR). Control remission induction therapy versus clofarabine treatment. Relapse-free survival was assessed in patients following attainment of a CR/CRi (upper panel). Probabilities for the endpoint relapse (middle panel) and nonleukemic death (ie, death in continued CR) (lower panel) are also plotted for the comparative study arms.
Relapse-free survival of complete responders and competing risk of relapse and death in complete remission (CR). Control remission induction therapy versus clofarabine treatment. Relapse-free survival was assessed in patients following attainment of a CR/CRi (upper panel). Probabilities for the endpoint relapse (middle panel) and nonleukemic death (ie, death in continued CR) (lower panel) are also plotted for the comparative study arms.
When we censor patients at the time of an alloSCT in the first CR (as an approach to analyzing the results in the absence of any possible contributory therapeutic effect of transplant on survival), no differences in the EFS and OS estimates between the control and clofarabine treatment groups became apparent either (data not shown).
Prognostic factors and subgroup analysis
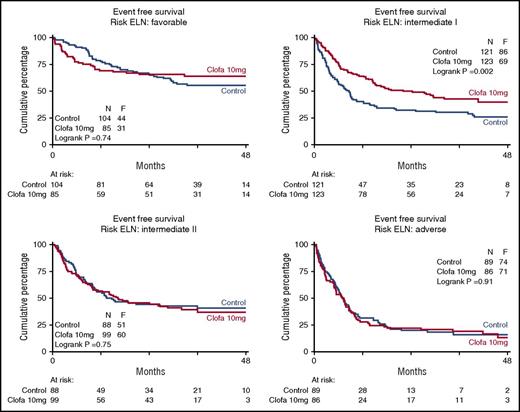
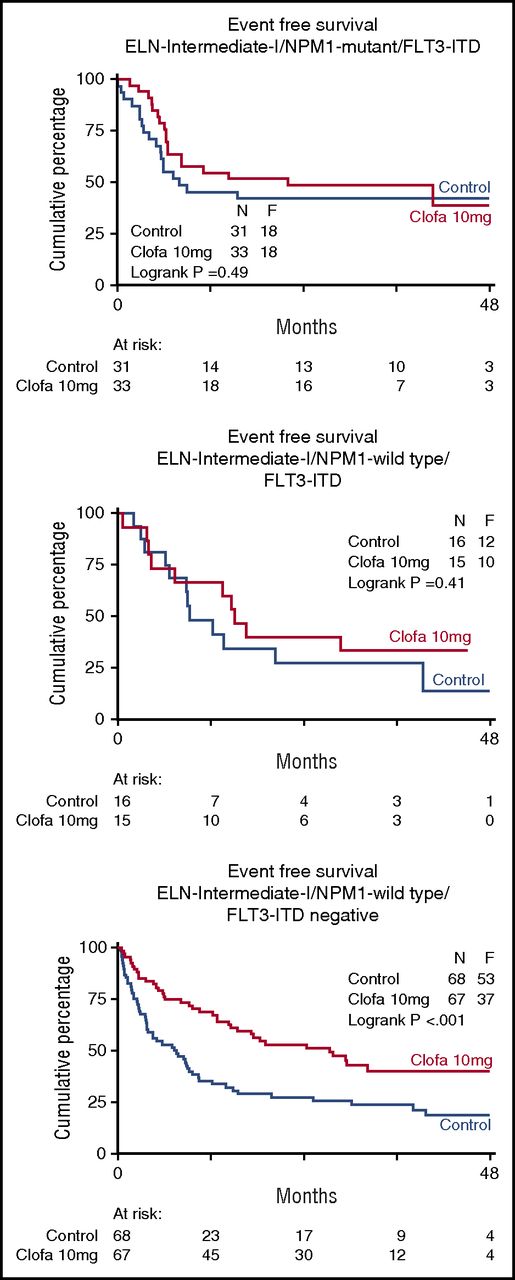
Table 3 shows the EFS and OS at 4 years split by treatment group and overall. In order to explore whether there was evidence indicative of a possible differential effect of clofarabine treatment in any of the subgroups defined by widely accepted prognostic factors for treatment outcome (age, WHO performance status, AML type [sAML, tAML, de novo, ELN 2010 prognostic risk category, as specified in the supplemental Appendix] and molecular genotypes), the effect of treatment was estimated by hazard ratios for EFS and OS, with associated confidence intervals within each of these subgroups (Table 3). The data indicate a favorable effect of the clofarabine regimen in the largest ELN intermediate I prognostic risk subset (n = 121 vs 123; EFS 26% ± 4 vs 40% ± 5; Cox P = .002; Figure 3; OS 29% ± 5 vs 50% ± 6; Cox P < .001; supplemental Figure 2). This positive effect of the clofarabine schedule on EFS and OS in part depended on a favorable effect in the molecular subset NPM1 wild-type/FLT3 without internal-tandem duplications (FLT3-ITD negative) (n = 68 vs 67; EFS, 18% ± 5 vs 40% ± 7; Cox P < .001; Figure 4; and OS, 22% ± 6 vs 49% ± 8; P < .001; supplemental Figure 3). When we include DNMT3A mutation (present/absent) in the analysis of the composite FLT3-NPM1 mutant/wild-type genotypes, the subsets with FLT3-ITD (irrespective of presence/absence of NPM1mutation) reveal an inferior survival outcome in case of additional DNMT3 mutations (DNMT3A-related survival data shown in supplemental Figure 4). Given the limited numbers, the latter data in the randomized treatment groups do not allow for a meaningful assessment of a possible effect of clofarabine treatment on survival of the distinct DNMT3A–FLT3-ITD–NPM1 genotypic subgroups. We also noted an advantage for clofarabine treatment of the subgroup of patients with the best WHO performance status PS0 (Table 3). The survival benefit of PS0 patients (in comparison with PS1-2) for the clofarabine group resulted from a significantly reduced relapse probability (EFS of patients with PS0 at 4 years, 36% ± 3 [controls] vs 44% ± 4 [clofarabine treatment], and relapse at 4 years of the PS0 subgroup, 40% ± 4 for controls vs 28% ± 3 for clofarabine treatment; EFS Cox P = .036).
Event-free survival of patients with distinct ELN prognostic risk scores. Control remission induction therapy versus clofarabine combination treatment. Favorable effect of clofarabine treatment with regard to EFS and OS (OS not shown) in ELN intermediate I risk group. ELN risk 2010 defined as ELN favorable, intermediate I, intermediate II, and adverse as described12 and slightly modified for CEBPA biallelic gene mutations as specified in the supplemental Appendix. The favorable effect of the clofarabine regimen on EFS is evident in ELN intermediate I risk group (n = 121 vs 123; EFS, 26% ± 4 vs 40% ± 5, Cox P = .002; OS, 29% ± 5 vs 50% ± 6, P < .001).
Event-free survival of patients with distinct ELN prognostic risk scores. Control remission induction therapy versus clofarabine combination treatment. Favorable effect of clofarabine treatment with regard to EFS and OS (OS not shown) in ELN intermediate I risk group. ELN risk 2010 defined as ELN favorable, intermediate I, intermediate II, and adverse as described12 and slightly modified for CEBPA biallelic gene mutations as specified in the supplemental Appendix. The favorable effect of the clofarabine regimen on EFS is evident in ELN intermediate I risk group (n = 121 vs 123; EFS, 26% ± 4 vs 40% ± 5, Cox P = .002; OS, 29% ± 5 vs 50% ± 6, P < .001).
Event-free survival of patients with genotypes based on common FLT3-ITD and NPM1 gene mutations. Control remission induction therapy versus clofarabine combination treatment. The favorable effect of the clofarabine regimen on EFS and OS (OS not shown) is evident in the comparatively larger molecular subset of AML of intermediate prognostic risk12 with nonmutated nucleophosmin-1 (NPM1) gene and absence of FLT3-ITD (Fms-like tyrosine kinase without internal tandem gene duplications) (n = 68 vs 67; EFS, 18% ± 5 vs 40% ± 7, Cox P < .001; OS, 22% ± 6 vs 49% ± 8, P < .001).
Event-free survival of patients with genotypes based on common FLT3-ITD and NPM1 gene mutations. Control remission induction therapy versus clofarabine combination treatment. The favorable effect of the clofarabine regimen on EFS and OS (OS not shown) is evident in the comparatively larger molecular subset of AML of intermediate prognostic risk12 with nonmutated nucleophosmin-1 (NPM1) gene and absence of FLT3-ITD (Fms-like tyrosine kinase without internal tandem gene duplications) (n = 68 vs 67; EFS, 18% ± 5 vs 40% ± 7, Cox P < .001; OS, 22% ± 6 vs 49% ± 8, P < .001).
Patients between 18 and 46 years of age (n = 220; 28% of patients) had similar EFS rates in both treatment groups, that is, 47% ± 5 (control) and 54% ± 5 (clofarabine treatment). Although treatment outcome overall for the older patients between 60 and 66 years of age (n = 215; 27% of patients in the study) was markedly less favorable, there were no differences in EFS following control and clofarabine treatments (EFS at 4 years, 17% ± 4 vs 24% ± 5 for the clofarabine treatment) (supplemental Table 1). Altogether, no differential benefit of the clofarabine schedule was apparent in patient subsets of variable age (≤45 years vs 46-60 years vs 61-65 years), nor in AML versus RAEB subgroups either (supplemental Table 1). Neither did we note any differences in outcome between both treatments in subgroups characterized by individual gene mutations in CEBPA (biallelic n = 26), NPM1 (n = 211), DNMT3A (n = 167), IDH1/IDH2 (n = 62/70), TP53 (n = 57), ASXL1 (n = 63), RUNX1 (n = 80), FLT3 tyrosine kinase domain (TKD845) (n = 48), FLT3-ITD (n = 139), SF3B1 (n = 13), SRSF2 (n = 55), KRAS (n = 40), NRAS (n = 129), PTPN11 (n = 69), or JAK2 (n = 15), but obviously most of these subgroups contain relatively limited numbers, perhaps too small to allow for a statistically sufficiently powerful analysis.
A multivariable analysis was performed to investigate the treatment effect, adjusting for the covariate’s age, WBC at diagnosis, ELN risk (2010,13 slightly modified according to the supplemental Appendix), and type of AML. In the multivariable Cox model the treatment effect for OS and RFS is not statistically significant, whereas the treatment effect of clofarabine in the multivariable Cox model for EFS is just significant (HR = 0.83, 95% confidence interval, 0.69-0.99) (further details are in Table 4).
Multivariable analysis for event-free survival
| Variable . | P . | Relative hazard ratio ± SE . | Confidence interval . |
|---|---|---|---|
| <.001 | |||
| Clofarabine treatment | .037 | 0.83 ± 0.08 | 0.69-0.99 |
| Age | <.001 | 1.03 ± 0.0 | 1.02-1.03 |
| White blood cell count | <.001 | 1.17 ± 0.04 | 1.10-1.25 |
| ELN intermediate risk I | <.001 | 2.10 ± 0.30 | 1.59-2.78 |
| ELN intermediate risk II | <.001 | 1.95 ± 0.30 | 1.45-2.63 |
| ELN adverse risk | <.001 | 3.99 ± 060 | 2.97-5.35 |
| Secondary AML | .0072 | 1.49 ± 0.22 | 1.11-1.98 |
| Treatment-related AML | .91 | 1.02 ± 022 | 0.68-1.55 |
| Variable . | P . | Relative hazard ratio ± SE . | Confidence interval . |
|---|---|---|---|
| <.001 | |||
| Clofarabine treatment | .037 | 0.83 ± 0.08 | 0.69-0.99 |
| Age | <.001 | 1.03 ± 0.0 | 1.02-1.03 |
| White blood cell count | <.001 | 1.17 ± 0.04 | 1.10-1.25 |
| ELN intermediate risk I | <.001 | 2.10 ± 0.30 | 1.59-2.78 |
| ELN intermediate risk II | <.001 | 1.95 ± 0.30 | 1.45-2.63 |
| ELN adverse risk | <.001 | 3.99 ± 060 | 2.97-5.35 |
| Secondary AML | .0072 | 1.49 ± 0.22 | 1.11-1.98 |
| Treatment-related AML | .91 | 1.02 ± 022 | 0.68-1.55 |
Result for the multivariable Cox regression for EFS. The following covariates were considered: patient age, white blood cell count at diagnosis (log transformation), ELN risk category 2010 (values expressed in relation to ELN favorable),12 secondary and treatment-related AML (in relation to AML de novo). ELN risk 2010 defined according to Döhner et al12 but slightly modified for bi-allelic CEBPA gene mutations, as detailed in the supplemental Appendix.
Adverse events, early death rate, death in CR, and hematological recovery
The 2 treatment arms were compared with respect to adverse events associated with cycles I and II (supplemental Table 2). In the clofarabine treatment group, more adverse effects of grades 3 to 4 and more infections were noted after cycles I and II.
Time to neutrophil or platelet recovery between the 2 groups did not differ after cycle I, but after cycle II both neutrophil and platelet regeneration were delayed in patients assigned to the clofarabine regimen (supplemental Table 2). In accordance with this effect, the interval from the start of cycle II toward the day of the last platelet transfusion was prolonged in the clofarabine group (median, 29 vs 21 days) as was the interval between start cycle II and the next cycle III (median, 55 vs 47 days). Patients of the clofarabine treatment arm also spent slightly more nights in the hospital (32 vs 28 days) after cycle II as well as after consolidation cycle III (median, 56 vs 52 days) (supplemental Table 2). Also the number of patients not proceeding to consolidation therapy in CR1/CRi1 was greater following clofarabine treatment (48 vs 22 patients). Thus, taken together, these data are consistent with the notion that the clofarabine regimen was associated with an increase in cumulative hematological toxicity that became apparent after cycle II.
There were no significant differences in 30-day and 60-day mortality rates, but a moderate increase of deaths in complete remission in the clofarabine group was apparent in the actuarial analysis at 4 years (Table 2). The slightly enhanced early mortality in the clofarabine group is also reflected in the early decline of the survival curve in the ELN 2010 good-risk subset (Figure 3). The death rates in CR1 at 4 years compared 15% (n = 48) versus 19% (n = 66) between control and clofarabine therapies. This difference was largely determined by the occurrence of more frequent fatal infections and slightly more fatal hemorrhages (4 vs 7) in the clofarabine treatment group.
Discussion
This article reports the first large phase III study with mature follow-up in a head-to-head comparison on the use of clofarabine integrated into intensive remission chemotherapy in newly diagnosed patients with AML younger than 66 years of age. On the basis of the results of the study, there can be little doubt that clofarabine is an active anti-AML therapeutic drug. The results of the study show the additive antileukemic effect of clofarabine on top of an anthracycline-cytarabine-based remission-induction program: earlier complete remissions were accomplished, and the probability of relapse was substantially reduced. However, the results also reveal an enhanced level of toxicity of the clofarabine-enforced combination of induction chemotherapy, which is evident from the increase in frequencies of serious adverse events and infections, a moderate increase in the number of deaths in remission (at 12 months), the delay in hematological recovery in particular after cycle II, and the prolonged interval between cycle II and start of consolidation cycle III. As a net result, overall EFS (primary study endpoint) and OS did not significantly improve as a consequence of the therapeutic clofarabine addition.
The most prominent prognostic factors for outcome in the subgroup and multivariate analysis were molecular and cytogenetic risk, the composite ELN risk 2010 classification, and age. None of the individual gene mutations for which we tested were associated with a therapeutic benefit for the clofarabine treatment, but it should be noted that the statistical power of these biomarker analyses was limited because of the fact that only moderate numbers were included in many of these subsets. An exploratory analysis revealed that patients with ELN intermediate risk I had much better outcome (EFS, OS) following treatment with the clofarabine regimen. The intermediate-risk molecular subset with a highly significant benefit among these patients was that with the NPM1 wild-type/FLT3-ITD negative genotype. In numerical terms these subgroups represent the comparatively largest subsets and permitted a more robust statistical comparison than did the other molecularly defined subsets. These data raise the question of whether these genotypically defined patients should receive clofarabine in future treatment management or whether additional confirmatory studies would be desired. It should be noted that the adverse-risk patients had no advantage of treatment intensification with the clofarabine adjunct. Thus it appears that there is an intermediate-risk group that is still susceptible to dose-level intensification with an active third drug. This phenomenon seems reminiscent of the pattern that has been described for the drug gemtuzumab ozogamicin (GO). GO has to be used at mitigated dose levels to avoid excess toxicity, and though it did not produce an overall survival advantage in newly diagnosed patients with AML, it has been suggested that the drug may confer a survival benefit in the chemosensitive AML subsets at the favorable and intermediate side of the prognostic risk spectrum of the disease.14-16 In the multivariable analysis a difference in survival (OS, EFS) between WHO performance status PS0 and PS1-2 became apparent. In addition, the apparent advantage of clofarabine is entirely restricted to PS0 patients and not apparent in PS1-2 patients. This selective survival benefit for PS0 patients is explained by the reduction of the relapse rate in the clofarabine treatment group (at 4 years’ relapse for PS0 patients, 40% ± 4 vs 28% ± 3; Cox P = .037). The death rate in CR1 at 48 months did not differ between PS0 (18% ± 3) and PS1-2 (19% ± 3) for the clofarabine treatment arm.
Moreover, we wish to note that in the HOVON-SAKK cooperative group studies, we have included patients with both AML and high-risk MDS (RAEB). In the current study we found no difference in outcome between both diagnostic categories, which lends support to offering a similar AML-based treatment approach to both AML and RAEB patients.
Although the current study yields reassurance that clofarabine is a potent AML compound, several questions remain. Could we have integrated clofarabine in a better way in the backbone of chemotherapy? Could we have done a better job by offering clofarabine in separate cycles rather than adding clofarabine on top of an already quite intensive chemotherapy schedule and thereby circumvent the increased toxicity issue? Or, analogous to the suggestive GO experience, could we have done a better job in circumventing the added toxicity with maintained antileukemic efficacy by applying a somewhat lower-dose level of the added drug (or maybe the backbone)? These questions, though relevant, cannot possibly be answered on the basis of current available information. All we can say is that we already used clofarabine at a lower dose of 10 mg/m2, lower than what has usually been used in previously published clofarabine combination studies.
Given the genomic heterogeneity of AML, there is an intense ongoing scientific effort to develop novel therapies targeted to genetically defined subsets of the disease. Studies in AML, such as the one reported here providing an unbiased approach across the breadth of various genotypes, may have particular value for identifying possible positive effects in specific AML subsets17 and thus pave the way for studies in distinct subtypes. The substantial difference in therapeutic outcome of the current phase III study may stimulate the interest in a specific study on clofarabine in intermediate risk AML.
In summary, the data reported here confirm the potent anti-AML efficacy of clofarabine. However, overall, the disadvantages of the enhanced toxicities offset the advantages of a reduction of relapse, so that ultimately there is no overall net benefit in survival in the broad population of patients with AML. The post hoc analysis suggests that there may be an advantage for the clofarabine schedule in an intermediate-risk subgroup among these patients (Figure 3; supplemental Figure 2). A similar positive effect with regard to EFS, OS, and RFS is apparent in the subgroup of AML with a NPM1 wild-type/FLT3-ITD negative genotype (Figure 4; supplemental Figure 3). Exploiting the potential therapeutic advantage of clofarabine in the first line of treatment in AML in an optimized way and exploring a less toxic scheduling of the drug will require additional study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the local and central data managers for collecting patient data, in particular Tamara van Dijk, Nicole Thuss, Petra Cornelisse, Annelies Verbrugge, Robby Sewsaran, Romy Lussenburg, Niek Lamers, and Rianne Ammerlaan (HOVON Data Center) and François Kavelaars for next-generation sequencing.
This investigator-sponsored trial was financially supported by Sanofi Pharma, and the drug clofarabine was provided free of charge.
Authorship
Contribution: The study was designed by the Leukemia Working Group of the HOVON/SAKK Cooperative Groups; the HOVON Data Center was responsible for the central data management; and Y.v.N. performed the analysis of the data. The decision to publish was made by the cooperative group. B.L. and subsequently Y.v.N. produced the first version of the manuscript, which was circulated for comments to the other authors.
Conflict of interest disclosure: The authors declare no competing financial interests.
A list of the members of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) appears in “Appendix.”
Correspondence: Bob Löwenberg, Department of Hematology (Ee1314), Erasmus University Medical Center, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: b.lowenberg@erasmusmc.nl.
Appendix: study group members
The participating member institutions/investigators of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) who participated in this study are: Belgium: Antwerp: Ziekenhuis Netwerk Antwerpen, D. A. Breems; Brussels: Hôpital St. Luc, A. Ferrant, V. Havelange, and M.C. Vekemans; Gent: University Hospital Ghent, E. Steel; Haine: Hôpital St. Paul Jolimont, A. Delannoy; Leuven: University Hospital Gasthuisberg, J. A. Maertens; Liege: Citadelle, B. Hodossy, S. Vansteenweghen, and L. Lammertijn; Montigny-le-tilleul: CHU Vésale, A. Triffet; Roeselare: AZ Delta, D. Deeren; Wilrijk: GasthuisZusters Antwerpen, C. Schuermans; Yvoir: CHU UCL Namur (Godinne), C. Graux and A. Sonet; Norway: Bergen: Haukeland University Hospital, B. T. Gjertsen; Sweden: Umeå: University Hospital North, A. Wahlin; Uppsala: Uppsala University Hospital, M. Höglund; Switzerland: Aarau: Kantonsspital, M. Bargetzi; Basel: University Hospital, J. Passweg and D. Heim; Bellinzona: San Giovanni, Georg Stuessi; Bern: Inselspital, T. Pabst; Fribourg: Kantonsspital, D. Betticher; Geneva: University Hospital, Y. Chalandon; Lausanne: University Hospital, O. Spertini; Luzern: Kantonsspital, M. Gregor; St. Gallen: Kantonsspital, U. Hess; Zurich: University Hospital, M. G. Manz; and The Netherlands: Amersfoort: Meander MC, S. K. Klein; Amsterdam: Academic Medical Center, B. J. Biemond; Amsterdam: OLVG, J. Terpstra and O. C. Leeksma; Amsterdam: VU University Medical Center, G.J. Ossenkoppele, A. van de Loosdrecht, and E. Meijer; Breda: Amphia, J. W. J. van Esser; Delft: Reinier de Graaf Gasthuis, R. E. Brouwer; The Hague: Hagaziekenhuis, D. van Lammeren-Venema; Dordrecht: Albert Schweitzer Ziekenhuis, M. D. Levin; Eindhoven: Maxima MC, L. W. Tick; Enschede: Medisch Spectrum Twente, M. C. J. C. Legdeur; Groningen: University Medical Center Groningen, E. Vellenga; Heerlen: Atrium MC, G. K. S. Jie; Leeuwarden: Medical Center Leeuwarden, M. Hoogendoorn; Leiden: Leiden University Medical Center, J. H. Veelken and P. A. von dem Borne; Maastricht: Maastricht University Medical Center, H. C. Schouten; Nieuwegein: St. Antonius Hospital, O. de Weerdt; Nijmegen: Radboud MC, G. Huls; Rotterdam: Erasmus University Medical Center, P. Sonneveld, J. Cornelissen, J. J. Zijlmans, M. Jongen-Lavrencic, G. E. De Greef, and B. Löwenberg; Utrecht: University Hospital Utrecht, J. Kuball and E. Petersen; Zwolle: Isala, M. Van Marwijk Kooy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal