Abstract
Research in the last few years has revealed a sophisticated interaction network between multiple bone marrow cells that regulate different hematopoietic stem cell (HSC) properties such as proliferation, differentiation, localization, and self-renewal during homeostasis. These mechanisms are essential to keep the physiological HSC numbers in check and interfere with malignant progression. In addition to the identification of multiple mutations and chromosomal aberrations driving the progression of myeloid malignancies, alterations in the niche compartment recently gained attention for contributing to disease progression. Leukemic cells can remodel the niche into a permissive environment favoring leukemic stem cell expansion over normal HSC maintenance, and evidence is accumulating that certain niche alterations can even induce leukemic transformation. Relapse after chemotherapy is still a major challenge during treatment of myeloid malignancies, and cure is only rarely achieved. Recent progress in understanding the niche-imposed chemoresistance mechanisms will likely contribute to the improvement of current therapeutic strategies. This article discusses the role of different niche cells and their stage- and disease-specific roles during progression of myeloid malignancies and in response to chemotherapy.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the American Nurses Credentialing Center (ANCC), the Accreditation Council for Pharmacy Education (ACPE), and the Accreditation Council for Continuing Medical Education (ACCME), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 920.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Associate Editor David M. Bodine and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Identify main niche alterations in acute myeloid leukemia and Philadelphia chromosome–negative myeloproliferative neoplasms, based on a review.
Identify main niche alterations in chronic myelogenous leukemia.
Identify main niche alterations in myelodysplastic syndrome.
Release date: February 16, 2017; Expiration date: February 16, 2018
Introduction
Myeloid malignancies are clonal hematopoietic disorders characterized by excessive proliferation, abnormal self-renewal, and/or differentiation defects of hematopoietic stem cells (HSCs) and myeloid progenitor cells. They mainly consist of myeloproliferative neoplasms (MPNs), myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML), caused by different genetic and epigenetic changes in HSCs and functional changes in bone marrow (BM) niche cells. Genetic and epigenetic modifications have also been noted in BM mesenchymal stromal cells (BMSCs) in MDS and AML. The end results of these alterations are phenotypically distinct diseases that likely require the design of specific treatments. However, the use of selective inhibitors is challenging, because they sometimes also affect the normal hematopoietic counterparts, and clones carrying other mutations often cause relapse after chemotherapy. In this case, overcoming common mechanisms of resistance might be more likely to succeed therapeutically.
The World Health Organization subdivided MPNs into four distinct diseases: chronic myelogenous leukemia (CML), characterized by the BCR-ABL oncogene fusion (Philadelphia chromosome [Ph+]) protein and the three Ph– disorders named polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1 Whereas PV is primarily associated with high erythrocyte counts, patients with ET have high platelet counts, and PMF is mainly related to BM failure as a result of fibrotic BM degeneration. These different clinical symptoms suggest that each MPN subtype is an independent disease, but transitions among them are observed in some patients.
Alterations of BM niches contribute to the progression of myeloid malignancies
Streaming from the discovery of driver mutations, myeloid malignancies were initially regarded as primarily driven by leukemic cell–autonomous mechanisms. However, cumulative evidence indicates that leukemic cells can exploit physiological niche signals and can overcome control by the normal microenvironment and/or remodel BM niches into permissive/self-reinforcing environments that support disease progression at the expense of normal hematopoiesis.
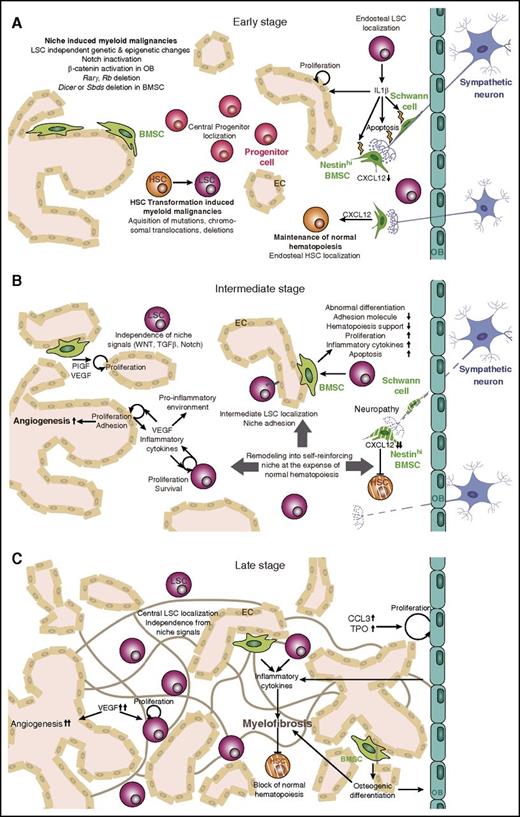
During leukemogenesis, malignant clones become progressively independent of niche-imposed physiological control mechanisms. In early leukemogenesis, BM homing and spatial localization of early leukemic stem cells (LSCs), which are also called pre-LSCs, are similar to that of normal HSCs. However, at later stages, LSCs home similarly to committed progenitors and become progressively independent of microenvironmental WNT signals.2 Some LSC alterations can simultaneously stimulate proliferation and myeloid skewing without affecting self-renewal. For instance, reduced JunB expression observed in many myeloid malignancies diminishes the responsiveness of LSCs to Notch and transforming growth factor β (TGF-β) niche signals.3 LSCs can become progressively insensitive to TGF-β during disease evolution from chronic to acute leukemia.4
The first indications of microenvironmental contribution to myeloid malignancies derive from reciprocal BM transplantation experiments showing that myeloid malignancies can arise from originally nonmutated hematopoietic cells in an altered microenvironment. For instance, MPN-like disease is observed in mice carrying a constitutive nonhematopoietic retinoic acid receptor γ deletion.5 MPN-like disease also develops after combined retinoblastoma protein deletion in nonhematopoietic and myeloid cells.6,7 Similarly, Notch pathway inhibition by the deletion of ubiquitin E3 ligase Mind bomb 1 (Mib1) in nonhematopoietic cells causes nontransplantable MPN-like disease, which can be reverted by microenvironmental Notch activation.8 Altogether, these pioneering studies represent strong evidence that the microenvironment exerts more than a mere bystander effect in myeloid malignancies.
Role of the BM vasculature
Several myeloid malignancies, including AML, MPN, and MDS, have been correlated with increased BM angiogenesis.9-13 BM vascularization in MPN patients correlates with janus kinase (JAK2) allele burden and stage, being highest in PMF, followed by CML, PV, and ET patients.11,12,14 MDS is characterized by lower vascularization, but blood vessel density similarly increases with disease aggressiveness and specifically correlates with progression to fibrosis.13,15 In addition to the increased vascular density, BM vessel morphology is disorganized and irregular in MPN and AML.11,16 Hence, increased and disorganized BM vascularization is a common niche alteration of myeloid malignancies.
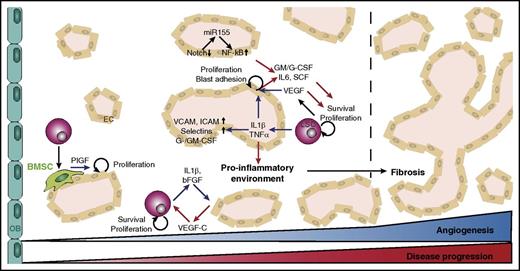
Progression of myeloid malignancies is supported by synergistic crosstalk between malignant blasts and endothelial cells (ECs). Enhanced BM vascularization correlates with upregulation of angiogenic factors, including vascular endothelial growth factor (VEGF)–A and interleukins (ILs).10,12,17-20 Whereas blasts secrete proangiogenic molecules, ECs release angiocrine factors that promote blast survival and proliferation.9,17 Blast-derived angiogenic factors act in a paracrine manner and also stimulate leukemic cell survival and proliferation via autocrine pathways.19,21,22 Similar to solid tumors, leukemic cells produce the key proangiogenic factor VEGF-A, which stimulates angiogenesis by paracrine mechanisms and increases blast survival and proliferation via autocrine VEGFR2-dependent pathways.17,19,21,23 Endothelial VEGF signaling stimulates blood vessel formation and also induces angiocrine factor (such as granulocyte-macrophage colony-stimulating factor [GM-CSF], macrophage CSF [M-CSF], granulocyte CSF [G-CSF], IL-6, and stem cell factor) production in ECs, which promotes proliferation of malignant cells (Figure 1).24-26 VEGF-dependent EC activation similarly increases EC-AML cell adhesion and AML aggressiveness.26
Role of BM blood vessels in myeloid malignancies. Angiogenesis increases during progression of myeloid malignancies and is particularly associated with fibrotic stages of the disease. Leukemic cells produce angiogenic factors such as VEGF and inflammatory cytokines (blue arrows) to stimulate proliferation of ECs, expression of adhesion molecules, and secretion of angiocrine factors. EC-derived angiocrine factors (red arrows) stimulate leukemic cell proliferation and survival, triggering a vicious cycle to remodel the BM into a self-reinforcing niche. OB, osteoblast.
Role of BM blood vessels in myeloid malignancies. Angiogenesis increases during progression of myeloid malignancies and is particularly associated with fibrotic stages of the disease. Leukemic cells produce angiogenic factors such as VEGF and inflammatory cytokines (blue arrows) to stimulate proliferation of ECs, expression of adhesion molecules, and secretion of angiocrine factors. EC-derived angiocrine factors (red arrows) stimulate leukemic cell proliferation and survival, triggering a vicious cycle to remodel the BM into a self-reinforcing niche. OB, osteoblast.
Leukemic cell–derived proangiogenic and proinflammatory factors such as IL-1β and basic fibroblast growth factor (bFGF) can stimulate ECs to release VEGF-C, thereby supporting blast survival and proliferation.22 Megakaryocytes represent an alternative VEGF source in MDS and might also stimulate angiogenesis in ET and PMF.13 High VEGF-A and VEGF-C plasma concentrations are associated with adverse prognosis in AML and CML, and VEGF-A levels correlate with MPN disease stage (PMF>PV>ET).10-12,18,27 Targeting VEGF signaling with bevacizumab has not been successful so far, but adjuvant treatment with different tyrosine kinase inhibitors (TKIs) in AML and MPN might normalize the microenvironment and eradicate malignant cells. However, patient response to therapy is heterogeneous and identifying susceptible subgroups is crucial.18,28-30
The angiopoietin 1 (ANG)/TIE signaling system—a master regulator of solid tumor angiogenesis—is also gaining recognition in myeloid malignancies. High ANG2 levels in AML patients do not correlate with changes in vessel density but may be of prognostic value because they correlate with improved survival when VEGF expression is low.31-33 Abnormal ANG/TIE signaling has been detected in ECs and also in leukemic cells.34,35 Autocrine ANG1/TIE2 signaling in blasts induces signal transducer and activator of transcription 1 (STAT1)/3/5/6 and ERK pathways, which support leukemic cell proliferation,34,36 and TIE2/IP-3 kinase signaling increases AML cell survival.35
Other proangiogenic factors such as bFGF and HGF are also upregulated in AML, CML, and MDS.10 Likewise, proinflammatory cytokines, including tumor necrosis factor α (TNF-α), IL-6, and IL-1β, are increased when AML blasts are cocultured with ECs. These cytokines stimulate EC proliferation and G-CSF and GM-CSF production, thereby promoting leukemic cell expansion.25,37 Secretion of TNF-α and IL-1β by AML blasts upregulates endothelial adhesion receptors such as selectins VCAM-1 and ICAM-1 to support vascular adhesion and proliferation.38 EC activation by inflammatory cytokines might compromise vascular integrity and favor thrombosis, further aggravating the proinflammatory environment.
Alterations in ECs might be a predisposing factor for the development of myeloid malignancies. MPN-like disease has also been observed in response to deletion of endothelial-specific Rbpj.39 Loss of endothelial Notch signaling upregulates microRNA 155 (miR-155), which de-represses nuclear factor κB (NF-κB) leading to G-CSF and TNF-α overexpression and myeloid expansion. The potential relevance of this pathway is emphasized by an increased miR-155 level in human PMF BM.39 CML cells and ECs also communicate via exosomes by shuttling miR-126 to downregulate VCAM-1 and CXCL12 in ECs and decrease CML adhesion and migration.40
Despite the release of multiple proangiogenic factors in the tumor microenvironment, hypoxia represents a common feature of myeloid malignancies and can influence LSC cycling, quiescence, differentiation, metabolism, and chemotherapy resistance. However, the role of hypoxia and downstream hypoxia-inducible factor 1α (HIF-1α) signaling in leukemia remains controversial, with published evidence for both supporting and inhibitory roles. In some studies, hematopoietic HIF-1α deletion promotes AML and MPN progression in mice.41,42 Similarly, combined deletion of HIF-1α and HIF-2α can accelerate AML initiation, but it is dispensable for disease maintenance.43 In contrast, other studies indicate that HIF-1α and HIF-2α support LSC survival by inducing p16 and p19 signaling and reducing reactive oxygen species (ROS) levels and endoplasmic reticulum stress, respectively.44,45 Hypoxia-induced VEGF production in a mouse model of CML correlates with increased clonogenicity, maintenance, repopulation capacity, and TKI resistance of BCR-ABL+ cells.46 Cytarabine and doxorubicin resistance is partly conferred by HIF-1α signaling, which induces quiescence in AML subclones by interfering with apoptosis and supporting survival signaling.47,48 Hypoxia might also favor leukemogenic niche metabolism and cytokine secretion. In addition to hypoxia, cytokines, interferon alfa, hormones, and genetic modifications can stimulate HIF-1α signaling, and their deregulation in a leukemic niche might similarly control this pathway in a hypoxia-independent manner.49 HIF-1α might be a prognostic marker for high-risk AML and CML patients and a valuable therapeutic target. These divergent results on the role of hypoxia in the LSC niche call for further studies on this particular aspect of environmental control.
Intense morphologic and functional remodeling of BM vessels has been observed in myeloid malignancies and generally result in increased but dysfunctional vasculature. A synergistic crosstalk is established between ECs and leukemic cells, which stimulates the growth of both. Increased permeability of an activated endothelium might also favor adhesion and mobilization of both inflammatory and leukemic cells, further aggravating inflammation, invasion of peripheral organs, and resistance (discussed below).
Role of BMSCs
Although initial in vitro studies did not observe major alterations in BMSCs, recent in vivo characterization has identified BMSCs as essential components of the HSC niche that are deregulated in a disease-specific manner in myeloid malignancies. The niche might have disparate roles in various myeloid malignancies, and these roles might also change during disease evolution (Figure 2).
Microenvironmental changes during leukemogenesis. In the BM niche, HSC function is tightly controlled by a specialized microenvironment comprising sympathetic neurons, BMSCs, OBs, and ECs. (A) During early stages of myeloid malignancies, HSPCs acquire genetic alterations that transform them into LSCs. These mutations also create a proinflammatory environment that damages sensitive elements of the microenvironment, such as Schwann cells and their associated nerve terminals. (B) During intermediate stages of the disease, the environment remodels into a self-reinforcing niche that interferes with normal hematopoiesis. LSCs become independent of niche signals and localize more centrally in the BM. MSCs acquire an abnormal phenotype, and angiogenesis increases as a result of high VEGF and cytokine levels. (C) Late stages of the disease are characterized by a proinflammatory environment and myelofibrosis, high blood vessel density, and central LSC localization. Rarγ, retinoic acid receptor γ; Rb, retinoblastoma protein; TPO, thrombopoeitin.
Microenvironmental changes during leukemogenesis. In the BM niche, HSC function is tightly controlled by a specialized microenvironment comprising sympathetic neurons, BMSCs, OBs, and ECs. (A) During early stages of myeloid malignancies, HSPCs acquire genetic alterations that transform them into LSCs. These mutations also create a proinflammatory environment that damages sensitive elements of the microenvironment, such as Schwann cells and their associated nerve terminals. (B) During intermediate stages of the disease, the environment remodels into a self-reinforcing niche that interferes with normal hematopoiesis. LSCs become independent of niche signals and localize more centrally in the BM. MSCs acquire an abnormal phenotype, and angiogenesis increases as a result of high VEGF and cytokine levels. (C) Late stages of the disease are characterized by a proinflammatory environment and myelofibrosis, high blood vessel density, and central LSC localization. Rarγ, retinoic acid receptor γ; Rb, retinoblastoma protein; TPO, thrombopoeitin.
The first evidence of a possible role for BMSCs in myeloid malignancies arose from studies that identified chromosomal abnormalities such as hypodiploidy and chromosomal translocations, duplications, and deletions in hematopoietic cells of patients with myeloid malignancies and also in BMSCs.50-56 These cytogenetic abnormalities have been observed in 30% to 70% of the BMSCs from patients with MDS or AML and are different from hematopoietic mutations in the same individuals.50,52 Yet stromal genomic alterations are associated with unfavorable prognostic chromosomal abnormalities in hematopoietic cells.51,52 Likewise, 55% of the BMSCs from patients with MDS showed abnormal karyotypes, and 57% of the BMSCs from patients with AML who had trisomy 8 and monosomy 7 in hematopoietic cells showed cytogenetic aberrations.54 Similarly, trisomy 8 mosaicism is associated with increased incidence of myeloid leukemia and MDS, and stromal cells in these patients favor leukemic cell proliferation.57 Chromosomal and epigenetic abnormalities in BMSCs from patients with MDS and AML have been linked to certain disease subtypes and distinctive gene-expression programs.53,56,58 These genetic abnormalities in BMSCs suggest enhanced genetic susceptibility and an active role of BMSCs in the progression of MDS or AML.
The functional consequences of these BMSC alterations are still debated. Several studies have noted normal differentiation, adhesion, expression and survival and the ability to support hematopoiesis ex vivo in BMSCs from patients with MDS, CML, or AML.50-52,54,59-62 In contrast, other studies showed abnormal differentiation, defective hematopoietic supportive capacity, reduced expression of adhesion molecules, increased apoptosis, and increased production of IL-1β and stem cell factor in BMSCs of patients with myeloid malignancies.55,56,63-75
These in vitro studies proposed some divergent views on BMSC alterations and contributions to disease, but more recent in vivo studies on several myeloid malignancies have reported altered BMSC growth and differentiation and the production of cytokine and HSC retention factor. Whether these BMSC alterations were predisposing or initiating factors for disease has remained elusive so far. The first evidence that alterations of BMSCs can drive myeloid malignancies arose from the deletion of the RNA processing enzyme Dicer1 in osteoprogenitor cells, which caused MDS-like disease with sporadic transformation to AML. The disease could be reverted by transplanting leukemic cells from mice with Dicer1-deleted osteoprogenitors into wild-type mice. Loss of DICER1 in osteoprogenitor cells (but not in mature osteoblasts) resulted in downregulation of the ribosome maturation protein SBDS, which is mutated in human Shwachman-Bodian-Diamond syndrome and is associated with congenital BM failure and leukemic predisposition.76 Reduced expression of DICER, DROSHA, and SBDS has been noted in BMSCs from patients with MDS,77 emphasizing the potential clinical relevance of these findings. A recent study has provided mechanistic insight on genotoxic stress caused by mutations in BMSCs, which can impair normal hematopoiesis and favor leukemogenesis. Loss of Sbds in BMSCs in mice recapitulates the characteristic osteoporosis found in human Shwachman-Bodian-Diamond syndrome. It also stimulates BMSC p53 signaling and secretion of the inflammatory molecules S100A8 and S100A9. S100A8/A9 activates toll-like receptor 4 on normal hematopoietic stem and progenitor cells (HSPCs), which leads to inflammatory damage, including hyperpolarized mitochondria, which triggers increased ROS production and DNA double-strand breaks. The potential relevance of niche S100A8/A9 expression in human leukemogenesis is emphasized by the correlation of S100A8/A9 expression in BMSCs and bone lining cells and the leukemic evolution of patients with MDS.78
Patients with Noonan syndrome often carry a mutation in the RAS signaling mediator PTPN11 and are at increased risk for developing childhood MPNs. A recent study has now shown that leukemogenic effects of activating PTPN11 mutations are not solely hematopoietic cell autonomous, but that PTPN11 mutations in BMSCs and osteoprogenitor cells can similarly drive MPN progression. Excessive CCL3 production by PTPN11-activated BMSCs results in the recruitment of monocytes to BMSCs, which hyperactivate HSCs by secreting inflammatory cytokines, including IL-1β, thereby exacerbating disease progression.79
Recent studies using mouse models of CML, MPNs, and AML demonstrate that specific BMSC-leukemic cell interactions are important for leukemogenesis (Table 1).16,80,81 In an inducible BCR-ABL mouse model, CML cells support BMSC proliferation and abnormal differentiation, which generate functionally altered and inflammatory osteoblasts. BMSCs in CML failed to maintain normal HSCs because of reduced Cxcl12 expression, favoring the expansion of less niche-dependent LSCs. Osteoblastic cells in CML secrete proinflammatory cytokines (IL-1β and TNF-α) that amplify disease progression by triggering myeloid cell proliferation and creating a self-reinforcing niche.81 CML cells also instruct BMSCs to secrete PIGF, which stimulates angiogenesis and promotes CML proliferation and metabolism, in part independently of BCR-ABL1 signaling.82
Main niche alterations in different myeloid malignancies
| Disease name . | . | Alteration . |
|---|---|---|
| AML | Disease phenotype | Hyperproliferation and impaired differentiation of HSC and myeloid progenitors |
| Acute rapidly progressing disease | ||
| Genetic LSC alterations | Chromosomal translocations, inversions, mutations in NPM1, CEBPA, KIT, RUNX1, FLT-IDT, or epigenetic factors | |
| Niche alterations | Neuropathy correlating with altered microenvironment16 | |
| PTH activation in osteoblasts accelerates AML.4 | ||
| CD44 and E-selectin are important for LSC niche adhesion and maintaining LSC primitive state.94,95,99 | ||
| β-catenin activation in osteoblasts stimulates Jag1 expression which activates Notch signaling in HSCs to induce AML.113,114 | ||
| Ph– MPN | Disease phenotype | Clonal HSC disorder with hyperproliferation and expansion of myeloid cells |
| Erythrocythemia (PV), thrombocythemia (ET), BM fibrosis (PMF) | ||
| Slow progression, chronic disease stage, possible transformation to AML | ||
| Genetic LSC alterations | Mutations in JAK2 (PV, ET, PMF), MPL (ET, PMF), CALR (ET, PMF) | |
| Niche alterations | LSCs secrete IL-1β, which damages Schwann cells and sympathetic nerve terminals causing early neuropathy. This results in apoptosis of nestin+ BMSCs, reduces Cxcl12 production, and results in thrombocytosis and fibrosis.80 | |
| Reduced osteoblast numbers at late stage of the disease.112 | ||
| Inactivation of Notch or deletion of retinoic acid receptor γ or retinoblastoma protein can cause niche-induced MPN.5,6,8 | ||
| PTPN11 activation in BMSCs induces CCL3-mediated monocyte recruitment and subsequent IL-1β–dependent HSC hyperactivation driving MPN progression.79 | ||
| CML | Disease phenotype | Clonal HSC disorder with hyperproliferation and expansion of myeloid cells |
| Slow progression, chronic disease stage, possible transformation to AML | ||
| Genetic LSC alterations | Chromosomal translocation resulting in BCR-ABL gene fusion | |
| Niche alterations | CML cells support BMSC proliferation and abnormal differentiation of osteoblasts into inflammatory cells which secrete proinflammatory cytokines that trigger myeloid progenitor and osteoblast expansion as well as stromal remodeling. CXCL12 is reduced in BMSCs favoring LSCs at the expense of HSC expansion.81 | |
| TGF-β and Notch cause stromal remodeling.81 | ||
| PTH activation in osteoblasts attenuates CML.4 | ||
| CML cells instruct BMSCs to secrete PIGF, which stimulates angiogenesis, CML proliferation, and metabolism.81 | ||
| CD44/E-selectin-dependent adhesion of LSCs to niche cells93,98 | ||
| Osteoblast expansion negatively regulates HSC and LSC proliferation.111 | ||
| MDS | Disease phenotype | Differentiation defects in HSCs |
| Pancytopenia, myelodysplasia | ||
| Slow progression, chronic disease, possible transformation to AML | ||
| Genetic LSC alterations | Chromosomal deletions, NUP98-HOXD13 fusion, mutations in RUNX1, CEBPA, EVI1, NPM1, RAS and splice and methylation factors | |
| Niche alterations | Dicer deletion in osteoprogenitors causes MDS.76 | |
| Leukemic cells can reprogram BMSCs into BMSCs of a malignant niche, which provide the important niche factors LIF, VEGF, IGF-BP2 and N-cadherin.83 | ||
| Abnormal WNT signaling causes proliferation defects in BMSCs because of increased cell senescence.84-86 | ||
| Increased osteogenic potential of BMSCs110 | ||
| p53-S100A8/9-TLR inflammatory signaling in BMSCs causes genotoxic stress in HSCs and supports MDS development.78 |
| Disease name . | . | Alteration . |
|---|---|---|
| AML | Disease phenotype | Hyperproliferation and impaired differentiation of HSC and myeloid progenitors |
| Acute rapidly progressing disease | ||
| Genetic LSC alterations | Chromosomal translocations, inversions, mutations in NPM1, CEBPA, KIT, RUNX1, FLT-IDT, or epigenetic factors | |
| Niche alterations | Neuropathy correlating with altered microenvironment16 | |
| PTH activation in osteoblasts accelerates AML.4 | ||
| CD44 and E-selectin are important for LSC niche adhesion and maintaining LSC primitive state.94,95,99 | ||
| β-catenin activation in osteoblasts stimulates Jag1 expression which activates Notch signaling in HSCs to induce AML.113,114 | ||
| Ph– MPN | Disease phenotype | Clonal HSC disorder with hyperproliferation and expansion of myeloid cells |
| Erythrocythemia (PV), thrombocythemia (ET), BM fibrosis (PMF) | ||
| Slow progression, chronic disease stage, possible transformation to AML | ||
| Genetic LSC alterations | Mutations in JAK2 (PV, ET, PMF), MPL (ET, PMF), CALR (ET, PMF) | |
| Niche alterations | LSCs secrete IL-1β, which damages Schwann cells and sympathetic nerve terminals causing early neuropathy. This results in apoptosis of nestin+ BMSCs, reduces Cxcl12 production, and results in thrombocytosis and fibrosis.80 | |
| Reduced osteoblast numbers at late stage of the disease.112 | ||
| Inactivation of Notch or deletion of retinoic acid receptor γ or retinoblastoma protein can cause niche-induced MPN.5,6,8 | ||
| PTPN11 activation in BMSCs induces CCL3-mediated monocyte recruitment and subsequent IL-1β–dependent HSC hyperactivation driving MPN progression.79 | ||
| CML | Disease phenotype | Clonal HSC disorder with hyperproliferation and expansion of myeloid cells |
| Slow progression, chronic disease stage, possible transformation to AML | ||
| Genetic LSC alterations | Chromosomal translocation resulting in BCR-ABL gene fusion | |
| Niche alterations | CML cells support BMSC proliferation and abnormal differentiation of osteoblasts into inflammatory cells which secrete proinflammatory cytokines that trigger myeloid progenitor and osteoblast expansion as well as stromal remodeling. CXCL12 is reduced in BMSCs favoring LSCs at the expense of HSC expansion.81 | |
| TGF-β and Notch cause stromal remodeling.81 | ||
| PTH activation in osteoblasts attenuates CML.4 | ||
| CML cells instruct BMSCs to secrete PIGF, which stimulates angiogenesis, CML proliferation, and metabolism.81 | ||
| CD44/E-selectin-dependent adhesion of LSCs to niche cells93,98 | ||
| Osteoblast expansion negatively regulates HSC and LSC proliferation.111 | ||
| MDS | Disease phenotype | Differentiation defects in HSCs |
| Pancytopenia, myelodysplasia | ||
| Slow progression, chronic disease, possible transformation to AML | ||
| Genetic LSC alterations | Chromosomal deletions, NUP98-HOXD13 fusion, mutations in RUNX1, CEBPA, EVI1, NPM1, RAS and splice and methylation factors | |
| Niche alterations | Dicer deletion in osteoprogenitors causes MDS.76 | |
| Leukemic cells can reprogram BMSCs into BMSCs of a malignant niche, which provide the important niche factors LIF, VEGF, IGF-BP2 and N-cadherin.83 | ||
| Abnormal WNT signaling causes proliferation defects in BMSCs because of increased cell senescence.84-86 | ||
| Increased osteogenic potential of BMSCs110 | ||
| p53-S100A8/9-TLR inflammatory signaling in BMSCs causes genotoxic stress in HSCs and supports MDS development.78 |
PTH, parathyroid hormone; TLR, toll-like receptor.
Progression of Ph– MPN also disrupts normal BMSC function, thus promoting disease progression. Proinflammatory cytokines produced by JAK2V617F hematopoietic cells, such as IL-1β, can cause local neuropathy and microenvironmental damage that leads to disease manifestation. In this inflammatory environment, MPN-associated neuropathy sensitizes nestin+ BMSCs to undergo apoptosis and reduces their HSC niche properties (including Cxcl12 expression). Genetic depletion of nestin+ cells can worsen myelofibrosis and thrombocytosis, which is also observed in mice lacking the β3-adrenergic receptor. In contrast, neural protection by neurotrophic factors or neural stimulation of the microenvironment by chronic treatment with β3-adrenergic agonists rescues nestin+ cells and improves Cxcl12 and IL-1β expression, neutrophilia, thrombocytosis, and myelofibrosis.80
Similar neuropathy-driven microenvironmental deregulation has been reported in AML.16 Yet the neuropathy might be comparatively less relevant in AML because neural interventions did not significantly affect leukemogenesis in that study. Whether neuropathy-driven microenvironmental changes are more broadly relevant in other hematologic malignancies remains to be investigated.
Reciprocal leukemic-niche interactions have also been highlighted in MDS. On one hand, patient-derived MDS cell engraftment is dependent on niche factors, such as LIF, VEGF, IGF-BP2, and N-cadherin. On the other hand, exposure of normal BMSCs to MDS renders them malignant-like, which highlights MDS-induced BMSC reprogramming.83 Alterations of WNT signaling in BMSCs have been associated with defective BMSC proliferation in MDS,84,85 partially because of the induction of senescence.86
Communication between BMSCs and MDS cells is partly mediated by extracellular vesicles.87 Exosome-mediated crosstalk between CML cells and human BMSCs triggers IL-8–dependent survival.88,89 Similarly, primary leukemic cells and cell lines release microvesicles containing RNAs that alter the secretion of niche-reprograming factors.90
Together, these studies highlight the role of BMSCs as key elements of predisposition, manifestation, and evolution of myeloid malignancies. Whereas functionally or genetically altered BMSCs increase inflammation and genotoxic stress, mutated hematopoietic cells critically compromise the normal function of BMSCs in the HSC niche, hampering normal hematopoiesis and favoring leukemogenesis. Whether these changes in BMSCs initiate disease in humans and/or select for particular mutated clones is a subject of intense research.
Anchoring of myeloid leukemic cells to their niches
Adhesion molecules are important for LSC engraftment and interaction with the niche. CD44 is a glycoprotein receptor for hyaluronan, selectins, and osteopontin. A specialized glycoform of CD44 named HCELL is a BM homing receptor.91 CD44 is overexpressed in CML,92 and homing and engraftment of CML and AML LSCs to their BM niches is much more dependent on CD44 compared with normal HSCs or B-cell acute lymphoblastic leukemia cells. Anti-CD44 treatment reduces CML incidence and AML burden in xenografts.93,94 In addition to directing LSC homing, CD44 also maintains LSCs in a primitive state,94,95 and high CD44 levels correlate with AML induction and relapse in AML mouse models.96,97
CD44 is a potent E-selectin receptor in CML, and E-selectin blockade can also reduce LSC numbers in CML.98 E-selectin is overexpressed on BM endothelium in AML, and antagonizing E-selectin can sensitize AML cells to chemotherapy.99 CD44 cooperates with other adhesion molecules, such as CD49d100 and integrin β1, which inhibit CML proliferation.101 Treatment with INF-α can restore impaired integrin β1–mediated adhesion of CML cells and inhibit their proliferation.102,103 This interaction might be relevant in other malignancies because integrin β1–mediated adhesion influences chemotherapy sensitivity in AML and increased fibronectin secretion in early fibrotic stages of MPN.104,105
Downregulation of BM Cxcl12 helps different myeloid malignancies16,80,106 and correlates with HSPC mobilization and extramedullary hematopoiesis. GCSF produced by CML cells decreases Cxcl12 expression by BMSCs and directly impairs normal hematopoiesis in an inducible BCR-ABL transgenic model. Long-term LSCs show reduced homing and retention in the BM because of increased G-CSF and decreased CXCL12 levels.106
Role of osteoblasts
Reduced trabecular bone mass in retinoic acid receptor γ– or retinoblastoma protein–deficient mice correlates with aggravated MPN, suggesting that endosteal niche alterations can promote MPN progression.5,6 The role of osteoblasts in leukemia progression seems to be disease specific, because constitutive parathyroid hormone receptor activation in osteoblasts increases bone remodeling and attenuates CML progression but stimulates MLL-AF9 AML progression. Increased bone remodeling in mice with constitutively active parathyroid hormone signaling causes TGF-β release from bone, reducing LSC proliferation and maintenance in CML but not in AML, probably because of reduced TGFBR1 expression or higher constitutive pSMAD2/3 signaling in AML.4 These differences suggest that the niche or niches might play different roles at various stages of leukemogenesis and/or in a disease-specific manner (Figure 2).
The increased osteogenic potential of BMSCs can contribute to PMF.109 Similarly, increased osteoblastic priming has been observed in BMSCs from childhood MDS.110 Osteoblasts also expand during the chronic phase of CML,81 when they negatively regulate normal and malignant HSC proliferation.111 This is reminiscent of the role of nestin+ BMSCs in Ph– MPN, in which depletion of nestin+ cells or their Cxcl12 production can accelerate MPN progression.80 Therefore, MPN preleukemic cells seem to retain sensitivity to normal cues from the microenvironment, and protecting the niche might be beneficial at this stage. In contrast, during the blast crisis of this disease, which resembles acute leukemia, osteoblasts are markedly reduced,112 suggesting that osteoblasts are differentially affected in AML and CML. AML has been associated with increased bone remodeling and accumulation of osteoblast-primed BMSCs, which do not seem to be able to mature into osteoblasts, correlating with decreased mineralized bone.16
Strikingly, expression of a constitutively activated form of β-catenin in osteoblasts might be sufficient for driving AML-like disease. Activated β-catenin signaling increases osteoblastic Jagged expression, leading to aberrant Notch signaling in HSCs. Inhibition of osteoblastic Notch signaling by Jagged deletion or pharmacologic treatment with γ-secretase inhibitors prevents AML development in mice.113 The same group has shown that 38% of patients with MDS, AML, or MDS with leukemic transformation have increased nuclear β-catenin in Runx2-expressing osteoblastic cells associated with increased Notch activity in CD34+ HSPCs.114 Osteoblasts are decreased in patients with MDS or AML, and osteoblast recovery correlates with better prognosis.115 Overall, this represents another example of potential mechanisms of niche-driven oncogenesis in myeloid malignancies.
The niche in response to chemotherapy
High-dose chemotherapy (HDC) is used to eradicate leukemic cells in AML and advanced MDS. Cytotoxic agents can damage the BM microenvironment and compromise niche function, regeneration, and maintenance of normal hematopoiesis (Figure 3). Chronic stromal damage by HDC is manifested by reduced BMSCs and CD44 expression in allogenic BM transplantation recipients, which is associated with slower hematopoietic recovery.116,117 Myelosuppression can cause endothelial regression that leads to a discontinuous, hemorrhagic endothelium accompanied by endothelial denudation. Sinusoidal vessels are particularly sensitive to irradiation, and subsequent EC regeneration via VEGFR2 signaling is critical for hematopoietic reconstitution.118 Chemotherapy-triggered BM sympathetic neuropathy can lead to loss of nestin+ cells and ECs, which interferes with hematopoietic regeneration. Neuroprotective agents have been reported to protect nerves from chemotherapy-induced injury and to support the survival of blood vessels and associated nestin+ cells, which leads to accelerated hematopoietic recovery.119 Osteoblasts are reduced after multiple rounds of chemotherapy, and osteoprogenitor numbers are decreased in response to HDC, eventually causing osteopenia.120,121 Adipocyte accumulation in aplastic BM might compromise niche function by negatively influencing hematopoietic recovery after myeloablation.122 Therefore, the damage inflicted by chemotherapy in the BM microenvironment can interfere with normal hematopoiesis and eventually result in BM failure.
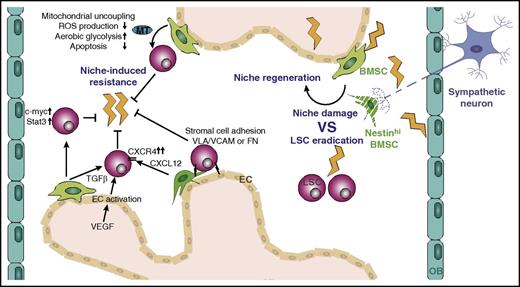
Protection of LSCs from chemotherapy by the microenvironment. Chemotherapy eradicates LSCs but at the same time damages multiple cell types of the niche and triggers subsequent niche regeneration. Prolonged treatment induces the development of resistance mechanisms, some of which are mediated by niche cells, including BMSCs and ECs. MT, mitochondria; FN, fibronectin.
Protection of LSCs from chemotherapy by the microenvironment. Chemotherapy eradicates LSCs but at the same time damages multiple cell types of the niche and triggers subsequent niche regeneration. Prolonged treatment induces the development of resistance mechanisms, some of which are mediated by niche cells, including BMSCs and ECs. MT, mitochondria; FN, fibronectin.
HSC transplantation used in relapsed or high-risk AML can rapidly induce neoplasia from malignant or premalignant donor HSCs.123 A dysfunctional host microenvironment resulting from mutations or HDC might also promote transformation of donor-derived HSCs into malignant cells. Likewise, growth of LSCs can alter the microenvironment and compromise normal HSC growth after allogenic HSC transplantation.
Although multiple LSC-intrinsic mechanisms of chemoresistance have been described, the microenvironment has recently attracted attention in protecting LSCs from chemotherapy (Figure 3). Early coculture studies showed that cytarabine treatment of BMSCs interferes with apoptosis and enhances survival of AML cells.124,125 BMSC-derived TGF-β1 is a mediator of resistance during cytarabine treatment of AML.126 Another key chemotherapy resistance–conferring pathway is CXCL12/CXCR4. Chemotherapy upregulates CXCR4 in AML cells, and imatinib enhances CXCR4 expression in BCR-ABL+ cells, which results in increased CXCL12/CXCR4 survival signaling and lodgment into protective niches.127-130 Adjuvant treatment of AML and CML with CXCR4 inhibitors decreases BMSC-induced survival pathways and sensitizes AML and CML cells to chemotherapy and imatinib treatment, respectively.127-130 Co-recruitment of CXCR4 and its downstream mediator Lyn into lipid rafts is another imatinib-induced chemotherapy resistance mechanism in CML.131 In fact, pharmacologic targeting of lipid rafts in combination with CXCR4/TGF-β1 can further sensitize CML cells to therapy.126,131
BMSC-induced CML chemotherapy resistance also occurs via upregulation of galectin-3, which stimulates leukemic cell proliferation, protection from apoptosis, and BM lodgment.132 Likewise, N-cadherin–dependent interaction of stromal and CML cells has been proposed to activate β-catenin signaling in CML cells, thereby shielding leukemic cells from TKI treatment.133 Reciprocal activation of NF-κB signaling via VCAM-1/very late antigen 4 (VLA-4) interaction occurs in BMSCs and AML cells, and blockade of stromal NF-κB signaling can sensitize AML cells to chemotherapy.134 Likewise, leukemic and stromal cell interaction via VLA-4 and fibronectin interferes with drug-induced apoptosis. Combined treatment of cocultures with VLA-4–specific antibodies and cytarabine improves survival, and patients with VLA-4–negative AML have a favorable prognosis.135 Human AML cells preferentially home and engraft in the endosteal BM of immunodeficient mice where they remain more quiescent and protected from chemotherapy.136
ECs can also confer chemotherapy resistance to AML cells, and blocking VEGFR2 signaling can increase the susceptibility of leukemic cells to chemotherapy.26,137 Leukemic cell adhesion to the vasculature has been proposed to induce quiescence, resistance to chemotherapy, and relapse.138 One study observed that AML cells acquired EC-like features and integrated into the blood vessel where they can become quiescent and evade chemotherapeutic treatment.139 Alternatively, ECs can protect AML cells from chemotherapy by producing high levels of VEGF and platelet-derived growth factor (PDGF) in response to cytarabine.140
Emerging evidence indicates that BMSCs shield LSCs from therapy by affecting their energy metabolism. Coculture of AML cells and BMSCs upregulates the mitochondrial proteins BCL2 and UCP2, which modify cellular energy metabolism by uncoupling leukemic mitochondria, suppressing ROS level, increasing the apoptotic threshold, and supporting aerobic glycolysis (Warburg effect). The increased apoptotic threshold resulting from decreased mitochondrial membrane potential and reduced ROS level can also protect LSCs from chemotherapy.141,142 A recent study has shown that BMSCs can modify LSC metabolism by directly transferring mitochondria to AML blasts in a cell-cell contact- and endocytosis-dependent manner. Mitochondrial uptake by the leukemic blasts increases their adenosine triphosphate production and protects them from mitochondrial depolarization after chemotherapy, thereby providing a survival advantage.143
BMSCs cocultured with AML cells promote chemotherapy resistance by increasing c-myc levels in AML cells, and c-myc inhibition can rescue AML cells from microenvironment-mediated drug resistance.144 Conditioned medium from BMSCs has been shown to support Stat3 survival signaling in CML cells in response to imatinib.145 Combining CML targeting by TKIs with the JAK2 inhibitor ruxolitinib can overcome resistance by blocking JAK/STAT signaling activated by BMSC-derived cytokines.146 Evidence for BMSC-induced chemotherapy resistance has been obtained mainly from studies on AML and CML, but other cytokines produced by BMSCs (including IL-6, FGF, and CXCL10) can also promote JAK2V617F+ cell resistance to atiprimod. Cytokine neutralizing antibodies may effectively restore apoptosis in Ph– MPN cells.147
Treating the AML subtype acute promyelocytic leukemia (APL) with all-trans-retinoic acid induces LSC differentiation and improves patient survival. Non-APL AML is unresponsive to differentiation therapy, and APL patients eventually relapse.148 Several leukemic cell–intrinsic resistance mechanisms have been identified, but upregulation of the all-trans-retinoic acid–metabolizing enzyme cytochrome P450 gene CYP26 by stromal cells contributes to development of minimal residual disease.149 Likewise, CYP3A4 upregulation in stromal cells confers resistance during etoposide treatment of AML.150
The understanding of niche-controlled resistance is still in its infancy, and despite the continuous development of novel treatment options, evading resistance mechanisms might arise. An important additional challenge is to avoid the elimination of normal HSCs. Therefore, identifying factors released by tumor cells that trigger resistance mechanisms conferred by niche cells is of importance to eventually interfere with disease relapse (Figure 3). Development of more selective drugs that act only on the mutated hematopoietic cells and/or combined treatment targeting not only the mutated cells but also the microenvironment might improve the outcome. However, the therapeutic strategies targeting the microenvironment should discriminate phases of normal HSPC niche damage vs advanced niche transformation. At early-stage disease and/or to diminish the damage caused by chemotherapy on normal niches, preventive strategies aiming at protecting the normal microenvironment might boost normal hematopoiesis and help to control preleukemic cells. However, at advanced disease stages, when the microenvironment has been profoundly transformed to support leukemogenesis, different niche-targeting strategies will be needed.
Future directions
Significant progress over the past few years has revealed important roles of the BM microenvironment in the pathogenesis of myeloid malignancies. However, the underlying mechanisms are only beginning to be elucidated, and an increasing complexity is becoming apparent, with different roles in distinct diseases and disease stages. Therefore, caution should be taken when extrapolating conclusions. Future focus on the complex interaction of neoplastic and microenvironmental cells will improve the development of niche-targeting strategies. Important questions remain for the future. For instance, do LSCs reside within specific niches? Do they interact with specific cell types? And are these cell types different from those interacting with normal HSCs? Do other cells regulating normal HSCs also play a role in leukemogenesis? Immune cells such as macrophages play key roles in the microenvironment of solid tumors and lymphoid malignancies, but their contribution to the myeloid malignancies remains much less explored. Do distinct niches for progenitor cells contribute to specific subtypes of malignancies? Are there key niche alterations during leukemogenesis leading to leukemic transformation (from preleukemia to leukemia)? Do somatic mutations in nonhematopoietic cells contribute to hematologic malignancies and how? Adjuvant therapies targeting the contribution of the microenvironment to leukemogenesis and resistance will likely be needed to fully eradicate LSCs. However, rational design of novel treatment strategies first requires proper understanding of the normal niches and their alterations in myeloid malignancies. Because the incidence of these diseases increases with age, parallel study of the aging process is of major importance. The reduced tolerance to chemotherapy, which contributes to the increased lethality of myeloid malignancies in the elderly, might be partially the result of impaired niche recovery during aging.
The current state-of-the art literature highlights the importance of the BM niche in contributing to myeloid malignancy progression by inducing or facilitating disease development, as well as conferring resistance to chemotherapy. The emerging recognition of the environment as a crucial player in multiple steps of the leukemic cascade lays the foundation for tackling leukemia from a different angle to improve current treatments.
Acknowledgments
The authors regret that some of the relevant literature had to be omitted because of space constrictions.
This work was supported by core support grants from Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute, National Health Service Blood and Transplant, and Marie Curie Career Integration Grant No. H2020-MSCA-IF-2015-708411 (C.K.) and Grant No. ERC-2014-CoG-64765 (S.M.-F.) from Horizon 2020.
Authorship
Contribution: C.K. prepared the figures and wrote the manuscript; and S.M.-F. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simón Méndez-Ferrer, University of Cambridge, National Health Service Blood and Transplant, Room F59, Cambridge Biomedical Campus, Long Rd, Cambridge CB2 0PT, United Kingdom; e-mail: sm2116@medschl.cam.ac.uk.