Abstract
Long noncoding RNAs (lncRNAs) are increasingly recognized as vital components of gene programs controlling cell differentiation and function. Central to their functions is an ability to act as scaffolds or as decoys that recruit or sequester effector proteins from their DNA, RNA, or protein targets. lncRNA-modulated effectors include regulators of transcription, chromatin organization, RNA processing, and translation, such that lncRNAs can influence gene expression at multiple levels. Here we review the current understanding of how lncRNAs help coordinate gene expression to modulate cell fate in the hematopoietic system. We focus on a growing number of mechanistic studies to synthesize emerging principles of lncRNA function, emphasizing how they facilitate diversification of gene programming during development. We also survey how disrupted lncRNA function can contribute to malignant transformation, highlighting opportunities for therapeutic intervention in specific myeloid and lymphoid cancers. Finally, we discuss challenges and prospects for further elucidation of lncRNA mechanisms.
Introduction
Lifelong generation of functionally specialized blood cells demands tight coordination of cell lineage specification, proliferation, and differentiation, requiring dynamic yet precise gene programming. Mounting evidence implicates many long noncoding RNAs (lncRNAs) in the assembly and targeting of multiprotein complexes that enact such programming. lncRNAs are broadly defined as >200 nt transcripts lacking functional coding capacity. Dozens of lncRNAs are known to modulate diverse processes, such as cell pluripotency, lineage commitment, and apoptosis,1-3 such that one unifying theme among functional lncRNAs is the regulation of cell fate. Here, we review lncRNAs that modulate cell fate in the hematopoietic system and synthesize their emerging mechanistic principles, reviewing in detail a subset of studies that have raised our understanding of their action modes during health (Table 1) and disease (Table 2).
Examples of mechanistically studied lncRNAs with roles during normal hematopoiesis
| Name . | Function . | Mechanism . | Reference . |
|---|---|---|---|
| Recruiter lncRNAs | |||
| LncHSC-2 | Modulates HSC self-renewal and differentiation | Mediates binding of TCF3 to active promoters of genes encoding hematopoietic developmental regulators | 47 |
| lincRNA-EPS | Represses apoptotic/inflammatory response genes | Diffuses to the promoters of its targets and establishes a repressive chromatin state, restraining their basal expression | 55,56 |
| linc-MAF-4 | Modulates T helper 1 differentiation | Recruits chromatin repressors to the neighboring MAF promoter to silence its transcription | 57 |
| Tmevpg1/NeST | Modulates helper T-cell activation | Recruits chromatin activators to the IFN-γ locus to promote its expression | 58 |
| Rmrp | Modulates T helper 17 cell differentiation | Forms a complex with DDX5 and RORγt and mediates its recruitment to genes that coordinate the Th17 effector program | 59 |
| lincRNA-Cox2 | Modulates innate immune response genes | Forms a complex with hnRNP-A/B and hnRNP-A2/B1 to repress transcription of immune response genes | 60 |
| THRIL | Modulates innate immune response genes | Recruits hnRNPL to the TNFα promoter to mediate its activation | 61 |
| Morrbid | Controls myeloid cell lifespan | Recruits chromatin repressors to the neighboring Bcl2l11 promoter to attenuate its transcription | 62 |
| alncRNA-EC7/Bloodlinc | Potentiates the terminal erythropoiesis gene program | Scaffolds chromatin and transcription regulators to modulate expression of loci encoding terminal erythropoiesis mediators | 34,35,66 |
| Decoy lncRNAs | |||
| NRON | Modulates NFAT localization/activation | Scaffolds NFAT and inhibitory kinases in the cytoplasm of resting cells, preventing activation and nuclear import | 68-70 |
| lnc-DC | Promotes the terminal dendritic maturation gene program | Sequesters active STAT3 away from inhibitory phosphatases, favoring nuclear import and activation of dendritic maturation genes | 36 |
| lnc-MC | Promotes monocyte/macrophage differentiation | Sequesters microRNA 199a-5p to mitigate repression of the ACVR1B monocyte/macrophage maturation regulator | 84 |
| PACER | Facilitates COX2 activation | Competes the repressive NF-κB1 homodimer away from the promoter of the neighboring COX2, favoring its induction | 74 |
| Lethe | Tunes NF-κB inflammatory responses | Titrates activating RELA away from target gene promoters, attenuating inflammatory gene activation | 76 |
| Name . | Function . | Mechanism . | Reference . |
|---|---|---|---|
| Recruiter lncRNAs | |||
| LncHSC-2 | Modulates HSC self-renewal and differentiation | Mediates binding of TCF3 to active promoters of genes encoding hematopoietic developmental regulators | 47 |
| lincRNA-EPS | Represses apoptotic/inflammatory response genes | Diffuses to the promoters of its targets and establishes a repressive chromatin state, restraining their basal expression | 55,56 |
| linc-MAF-4 | Modulates T helper 1 differentiation | Recruits chromatin repressors to the neighboring MAF promoter to silence its transcription | 57 |
| Tmevpg1/NeST | Modulates helper T-cell activation | Recruits chromatin activators to the IFN-γ locus to promote its expression | 58 |
| Rmrp | Modulates T helper 17 cell differentiation | Forms a complex with DDX5 and RORγt and mediates its recruitment to genes that coordinate the Th17 effector program | 59 |
| lincRNA-Cox2 | Modulates innate immune response genes | Forms a complex with hnRNP-A/B and hnRNP-A2/B1 to repress transcription of immune response genes | 60 |
| THRIL | Modulates innate immune response genes | Recruits hnRNPL to the TNFα promoter to mediate its activation | 61 |
| Morrbid | Controls myeloid cell lifespan | Recruits chromatin repressors to the neighboring Bcl2l11 promoter to attenuate its transcription | 62 |
| alncRNA-EC7/Bloodlinc | Potentiates the terminal erythropoiesis gene program | Scaffolds chromatin and transcription regulators to modulate expression of loci encoding terminal erythropoiesis mediators | 34,35,66 |
| Decoy lncRNAs | |||
| NRON | Modulates NFAT localization/activation | Scaffolds NFAT and inhibitory kinases in the cytoplasm of resting cells, preventing activation and nuclear import | 68-70 |
| lnc-DC | Promotes the terminal dendritic maturation gene program | Sequesters active STAT3 away from inhibitory phosphatases, favoring nuclear import and activation of dendritic maturation genes | 36 |
| lnc-MC | Promotes monocyte/macrophage differentiation | Sequesters microRNA 199a-5p to mitigate repression of the ACVR1B monocyte/macrophage maturation regulator | 84 |
| PACER | Facilitates COX2 activation | Competes the repressive NF-κB1 homodimer away from the promoter of the neighboring COX2, favoring its induction | 74 |
| Lethe | Tunes NF-κB inflammatory responses | Titrates activating RELA away from target gene promoters, attenuating inflammatory gene activation | 76 |
Examples of mechanistically studied lncRNAs with roles during malignant hematopoiesis
| Name . | Function . | Mechanism . | Reference . |
|---|---|---|---|
| Recruiter lncRNAs | |||
| Xist | Directs and maintains female X chromosome dosage compensation | Scaffolds chromatin repressors as it spreads along its chromosome, silencing most of its genes and compacting it into a repressive compartment | 54 |
| LUNAR1 | Sustains oncogenic IGF1R activation | Recruits transcription machinery to an enhancer element shared with the IGF1R gene | 65 |
| Decoy lncRNAs | |||
| GAS5 | Modulates T-cell growth arrest and apoptosis | Sequesters the glucocorticoid receptor by binding to its DNA-binding domain via a hairpin structure that acts as a decoy glucocorticoid response element | 3,72,103 |
| FAS-AS1 | Suppresses production of soluble FAS receptor | Sequesters RBM5 from the Fas pre-mRNA to prevent its alternative splicing into an isoform encoding a soluble decoy receptor | 77 |
| Name . | Function . | Mechanism . | Reference . |
|---|---|---|---|
| Recruiter lncRNAs | |||
| Xist | Directs and maintains female X chromosome dosage compensation | Scaffolds chromatin repressors as it spreads along its chromosome, silencing most of its genes and compacting it into a repressive compartment | 54 |
| LUNAR1 | Sustains oncogenic IGF1R activation | Recruits transcription machinery to an enhancer element shared with the IGF1R gene | 65 |
| Decoy lncRNAs | |||
| GAS5 | Modulates T-cell growth arrest and apoptosis | Sequesters the glucocorticoid receptor by binding to its DNA-binding domain via a hairpin structure that acts as a decoy glucocorticoid response element | 3,72,103 |
| FAS-AS1 | Suppresses production of soluble FAS receptor | Sequesters RBM5 from the Fas pre-mRNA to prevent its alternative splicing into an isoform encoding a soluble decoy receptor | 77 |
Characteristics of lcRNAs
Discovery and characterization of lncRNAs have been largely driven by the development of technologies for detecting and cataloguing entire transcriptomes (see Rinn and Chang,4 Ulitsky and Bartel,5 and Hu et al6 ; supplemental Text 1, available on the Blood Web site). These revealed that, like messenger RNAs (mRNAs), lncRNAs are often capped, polyadenylated, and spliced, yet most are expressed at lower levels and in a more tissue- and cell type–specific manner, than most mRNAs.7-9 Many lncRNAs are highly conserved, including ∼100 families conserved between fish and tetrapods, and >1000 families conserved across placental mammals.10,11 However, the majority of lncRNAs seem to be species- or lineage-specific, such that, for each species examined, most of its lncRNAs cannot be traced to species that diverged more than 50 million years ago.9-12 Like protein-coding genes, lncRNAs comprise several families with common properties and functions; they can be classified as intergenic, antisense to protein-coding loci, or overlapping known noncoding regions (eg, enhancers, introns of protein-coding genes, pseudogenes, small noncoding RNA loci). Regardless of their positioning, lncRNAs can originate from their own promoters, from promoters shared with divergently transcribed coding or noncoding genes, or from enhancer elements. Enhancer-driven lncRNAs, interestingly, may account for >50% of all intergenic lncRNAs detected in a given cell type.13,14
lncRNAs are regulated by the same chromatin and transcription factors (TFs) as mRNAs, although initiation and elongation of lncRNAs transcribed from divergent promoters is additionally modulated by factors that control promoter directionality, such as chromatin remodelers.15-17 Structurally, lncRNAs have exons of comparable size to those of mRNAs but tend to have fewer of them, resulting in shorter transcripts and fewer alternative isoforms. Splicing of lncRNAs occurs through canonical splice sites, but appears to be less efficient than for mRNAs.9,18
Some lncRNAs act in cis, affecting expression only of neighboring genes. Others diffuse from their site of transcription and act in trans, affecting genes located on different chromosomes. Unlike mRNAs, which specifically localize to the cytoplasm, lncRNAs can occupy various nuclear compartments (eg, chromatin, nucleoplasm, subnuclear domains), the cytoplasm, or both nuclear and cytoplasmic compartments.9,19,20 Whereas mRNAs are primarily degraded by cytoplasmic decapping enzymes and 5′-to-3′ exonucleases, many unstable lncRNAs are degraded by the nuclear exosome or by cytosolic nonsense-mediated decay (NMD) machinery.21-23 Of course, exceptions to each of the class properties described here exist, including lncRNAs that are larger than 100 kb, not polyadenylated, and that rival mRNAs in copy number. Importantly, studies over the past 2 decades combining cell-based and in vivo lncRNA loss- and gain-of-function assays have greatly expanded the number of lncRNAs with recognized functions (see Rinn and Chang4 and Amaral et al24 ; supplemental Text 2).
lncRNAs potentiate developmental programming
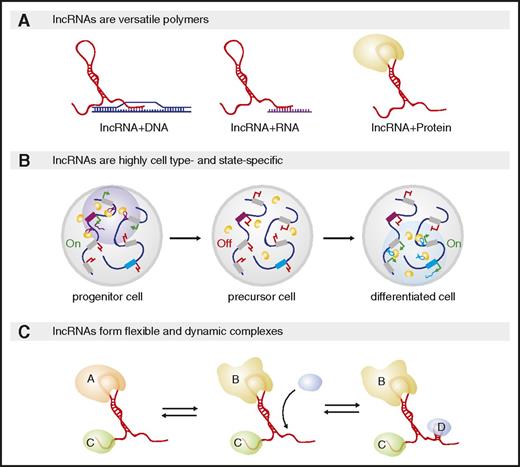
Cell lineage specification involves concurrent activation of specific cell fates and repression of alternative ones, programmed by transcription and chromatin factors that act as intrinsic lineage determinants. These factors are redeployed across distinct lineages and developmental stages, however, such that lineage choice reflects the particular combination of factors that interact at a given developmental stage. For example, during hematopoiesis, high-level GATA1 expression specifies the bipotential megakaryocyte/erythroid progenitor, and choice of the megakaryocytic or erythroid fate depends on whether GATA1 interfaces with FLI1 or EKLF/KLF1, respectively.25 Several features make lncRNAs well-suited for coordinating the assembly, localization, and targeting of cell fate–specifying complexes (Figure 1).
Unique features enable lncRNA-mediated control of cell fate and function. (A) lncRNAs can bind nucleic acids via base-pairing or proteins through secondary structures. The same lncRNA can harbor multiple interaction modules, enabling it to spatially organize diverse effectors and their targets and to scaffold ribonucleoprotein complexes. (B) lncRNAs are highly tissue-, developmental-, and physiological state-specific. Shown are 2 loci encoding lncRNAs (purple and blue boxes); gray boxes encode other transcripts. During early development (left), 1 lncRNA (purple transcripts) can form distinct nuclear compartments by recruiting a regulatory protein (yellow circles) to genomic sites surrounding its own locus. That lncRNA may be turned off at a later developmental stage whereas a second (blue transcript) is induced and recruits the same yellow protein but to different chromosome loci. (C) lncRNA–protein complexes are dynamic and flexible. The same lncRNA can bind distinct proteins based on their local concentration or by adopting distinct structural conformations with distinct RNA–protein binding affinities.
Unique features enable lncRNA-mediated control of cell fate and function. (A) lncRNAs can bind nucleic acids via base-pairing or proteins through secondary structures. The same lncRNA can harbor multiple interaction modules, enabling it to spatially organize diverse effectors and their targets and to scaffold ribonucleoprotein complexes. (B) lncRNAs are highly tissue-, developmental-, and physiological state-specific. Shown are 2 loci encoding lncRNAs (purple and blue boxes); gray boxes encode other transcripts. During early development (left), 1 lncRNA (purple transcripts) can form distinct nuclear compartments by recruiting a regulatory protein (yellow circles) to genomic sites surrounding its own locus. That lncRNA may be turned off at a later developmental stage whereas a second (blue transcript) is induced and recruits the same yellow protein but to different chromosome loci. (C) lncRNA–protein complexes are dynamic and flexible. The same lncRNA can bind distinct proteins based on their local concentration or by adopting distinct structural conformations with distinct RNA–protein binding affinities.
First, lncRNAs are versatile polymers that can bind nucleic acids via base-pairing or proteins through RNA secondary structures. As such, lncRNAs can physically bring together chromatin targets, creating structural domains.26,27 lncRNAs can also bind RNA targets, nucleating RNA compartments,28,29 and protein targets, forming ribonucleoprotein complexes.2,4 Because these effectors include regulators of chromatin organization and transcription or of RNA processing, translation, and decay, lncRNAs can regulate gene expression at multiple levels.30,31
Second, most lncRNAs are, unlike most proteins, expressed in only 1 or a few specific tissues and developmental and physiological states.7-9 Among blood cells, lncRNAs are highly specific to individual lineages and to physiological contexts such as the immune response.32,33 For example, alncRNA-EC7/Bloodlinc, which is essential for red cell maturation, and lnc-dendritic cell (DC), which is vital for DC differentiation, are expressed in erythroid and DCs only, respectively.34-36 Accordingly, distinct lncRNA assemblages are expressed in distinct lineages to support their development and function (reviewed in Hu et al6 and Fatica and Bozzoni37 ).
Third, lncRNAs can act as flexible scaffolds to facilitate dynamic gene control. Unlike proteins, which must be imported into the nucleus before they can reach gene targets, lncRNAs can function immediately upon synthesis. Accordingly, lncRNAs can be dynamically deployed in response to developmental/physiological changes to regulate gene expression by bringing together proteins and specific DNA targets into nuclear subcompartments.29,38-40 Rapid assembly or disassembly of such compartments needs only synthesis or degradation of the lncRNA, whereas their maintenance requires continued lncRNA production. For example, maintaining X chromosome dosage compensation in female hematopoietic stem cells (HSCs) requires the lncRNA Xist, which is transcribed from 1 of the 2 X chromosomes and directs its inactivation by recruiting transcription- and chromatin-repressive complexes (see the following section).41 lncRNA-nucleated complexes are also intrinsically flexible because lncRNAs can adopt distinct stable conformations to bind distinct proteins.42,43 Thus, lncRNAs can assemble unique combinations of multiprotein complexes that otherwise cannot interact with each other.
These features of lncRNAs, their versatility of interactions, cell type– and state-specificity, and dynamic and flexible behavior, enable them to act as modular adaptors that integrate combinatorial inputs into context-specific regulatory outputs. Exploiting this capacity thus enables metazoans to program a variety of specialized cell functions. In the next sections, we focus on lncRNAs that help enact cell type–specific regulatory programs among blood cells via RNA-based contributions, aiming to synthesize salient mechanistic principles. For brevity, we selected representative lncRNAs illustrating such principles that reflect only a subset of mechanistically studied lncRNAs during normal (Table 1) and malignant (Table 2) hematopoiesis. Readers wishing to explore the current breadth of functional lncRNAs in hematopoiesis are directed to Jeong and Goodell44 and Nobili et al45 for up-to-date catalogs.
Many lncRNAs scaffold proteins to their targets
All blood cell lineages derive from a single HSC. Recent evidence indicates that lncRNAs are highly integrated components of the pathways controlling HSC proliferation and differentiation.46-48 LncHSC-2 is an enhancer-derived nuclear lncRNA that tunes HSC self-renewal and differentiation seemingly by regulating the activity of TCF3/E2A,48 a TF required for HSC self-renewal and for maturation of myelo-lymphoid progenitors.49 LncHSC-2 acts in trans and binds active promoter-proximal regions of distant genes encoding proteins linked to hematopoietic phenotypes, including PML (essential for HSC maintenance) and ITPKB (critical for T-cell development), although how this affects transcription of these genes has not been evaluated. Interestingly, LncHSC-2 depletion in stem/progenitor cells followed by in vivo transplantation causes impaired HSC self-renewal and increased T-cell output. LncHSC-2 DNA-binding sites are also enriched for binding of TCF3, and TCF3 recruitment to these sites is abrogated in LncHSC-2–deficient cells. Whether LncHSC-2 directly recruits TCF3 (or other regulators) to these sites, however, and the molecular relevance of such associations, remain to be investigated.
Hematopoietic lineage commitment is determined by cross-antagonistic TFs that favor alternative developmental programs, such that their relative concentrations specify lineage fate.50 Because some of these TFs are encoded by the X chromosome, proper X-chromosome dosage in females, which requires the Xist lncRNA, is vital for normal hematopoiesis. Conditional Xist deletion in mouse HSCs is lethal for homozygous or heterozygous females.47 Xist−/− HSCs show widespread reactivation of X-encoded genes, including increased expression of GATA1, which specifies the myelo-erythroid fate in short-term repopulating HSCs.51 Accordingly, Xist−/− females undergo myelofibrosis, myeloproliferation, and myelodysplasia, and succumb to chronic myelomonocytic leukemia and erythroleukemia. This recapitulates the course of human mixed myelodysplastic/myeloproliferative neoplasms (MDS/MPN).52 Importantly, Xist-deficient HSCs show impaired maturation and loss of long-term repopulation ability, and wild-type mice transplanted with Xist−/− bone marrow develop mixed MDS/MPN, whereas Xist−/− mice transplanted with wild-type bone marrow do not, evidencing an HSC-autonomous defect. These findings link the Xist locus to the maintenance of X-chromosome dosage in vivo, and implicate it in the pathogenesis of MDS/MPN. Although MDS/MPN is rare, there is currently no treatment consensus for these diverse stem cell malignancies.52 Their challenging diversity may reflect broad X-chromosome overdosage, as noted in MDS, MPN, and myeloid cancers.53,54 Manipulating Xist function hence represents an attractive opportunity for therapeutic intervention in MDS/MPN.
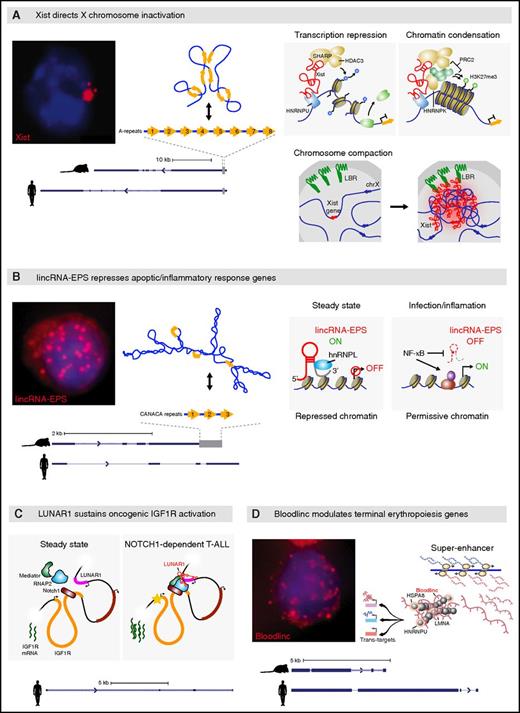
Xist’s mechanism is among the better understood lncRNA mechanisms to date (Figure 2A).41 Upon transcription from 1 of the 2 female X chromosomes during early embryogenesis, Xist spreads in cis and orchestrates a sequence of events, including loss of transcription-permissive chromatin marks, gain of transcription-repressive ones, and chromosome compaction, leading to silencing of most genes along its chromosome. Xist cis-anchoring depends on binding the chromatin attachment factor HNRNPU/SAFA,55 and its spreading pattern exploits 3-dimensional physical proximity, such that Xist migrates first to sites proximal to its transcription site.56,57 Transcription repression of these loci depends on Xist binding SPEN/SHARP/MINT through its A-repeat domain.58-60 SPEN is thought to recruit histone deacetylases in turn, leading to loss of H3K4me3 followed by RNA polymerase II exclusion.
Many lncRNAs scaffold proteins to their targets. (A) Xist directs X chromosome inactivation across eutherian females. RNA fluorescence in situ hybridization (FISH) image shows focal retention of Xist transcripts (red; blue fluorescence demarks the nucleus). Transcript models from mouse and human are shown at bottom. The gray bar at the right marks the A-repeat region, with the 8 tandem A-repeats depicted above. Base pairing among the repeats is stochastic, and one of the specific conformations they adopt in vivo is shown at top. Xist remains cis-tethered to DNA (blue line) by binding the chromatin attachment factor HNRNPU. Binding to SPEN via the A-repeat domain followed by recruitment of histone deacetylase 3 (HDAC3), which catalyzes H3K4 demethylation leading to transcription machinery exclusion, enables transcription repression. Xist also binds HNRNPK, which facilitates scaffolding of epigenetic repressors such as PRC2, which deposits H3K27me3, leading to a transcription-repressive chromatin state. As Xist spreads in cis, it binds the lamin B receptor (LBR) to reposition its chromosome to the nuclear lamina, forming a compact repressive compartment.41 (B) lincRNA-EPS represses apoptotic/inflammatory response genes. RNA FISH image shows nuclear diffusion of lincRNA-EPS transcripts (red). Transcript models from mouse and human are shown at bottom. The gray bar marks a 3′ region with tandem CANACA repeats, depicted at top, which is not conserved in humans. Its predicted secondary structure, comprising the minimum free energy conformation identified by RNAfold, is shown at top. Under steady-state conditions, lincRNA-EPS binds HNRNPL via the CANACA-repeat domain and localizes to promoters of target genes, conferring a repressed chromatin state by promoting nucleosome occupancy upstream of their transcription start site. This basal transcription repression is lifted during infection/inflammatory responses. (C) LUNAR1 sustains oncogenic IGF1R activation in cis. Aberrantly activated NOTCH1 engages an enhancer element intronic to the IGF1R gene (orange) which activates LUNAR1, which in turn cooccupies this element and favors recruitment/retention of the transcription machinery, including Mediator, leading to enhanced activation of the IGF1R promoter and accumulation of additional IGF1R transcripts. (D) Bloodlinc potentiates the terminal erythropoiesis gene program. RNA FISH image shows nuclear diffusion of Bloodlinc molecules. Transcript models from mouse and human are shown at bottom. Bloodlinc diffuses from a super-enhancer domain to trans-loci encoding terminal erythropoiesis modulators, and binds HNRNPU as well as transcription coactivators/corepressors, mediating activation or repression of its targets.
Many lncRNAs scaffold proteins to their targets. (A) Xist directs X chromosome inactivation across eutherian females. RNA fluorescence in situ hybridization (FISH) image shows focal retention of Xist transcripts (red; blue fluorescence demarks the nucleus). Transcript models from mouse and human are shown at bottom. The gray bar at the right marks the A-repeat region, with the 8 tandem A-repeats depicted above. Base pairing among the repeats is stochastic, and one of the specific conformations they adopt in vivo is shown at top. Xist remains cis-tethered to DNA (blue line) by binding the chromatin attachment factor HNRNPU. Binding to SPEN via the A-repeat domain followed by recruitment of histone deacetylase 3 (HDAC3), which catalyzes H3K4 demethylation leading to transcription machinery exclusion, enables transcription repression. Xist also binds HNRNPK, which facilitates scaffolding of epigenetic repressors such as PRC2, which deposits H3K27me3, leading to a transcription-repressive chromatin state. As Xist spreads in cis, it binds the lamin B receptor (LBR) to reposition its chromosome to the nuclear lamina, forming a compact repressive compartment.41 (B) lincRNA-EPS represses apoptotic/inflammatory response genes. RNA FISH image shows nuclear diffusion of lincRNA-EPS transcripts (red). Transcript models from mouse and human are shown at bottom. The gray bar marks a 3′ region with tandem CANACA repeats, depicted at top, which is not conserved in humans. Its predicted secondary structure, comprising the minimum free energy conformation identified by RNAfold, is shown at top. Under steady-state conditions, lincRNA-EPS binds HNRNPL via the CANACA-repeat domain and localizes to promoters of target genes, conferring a repressed chromatin state by promoting nucleosome occupancy upstream of their transcription start site. This basal transcription repression is lifted during infection/inflammatory responses. (C) LUNAR1 sustains oncogenic IGF1R activation in cis. Aberrantly activated NOTCH1 engages an enhancer element intronic to the IGF1R gene (orange) which activates LUNAR1, which in turn cooccupies this element and favors recruitment/retention of the transcription machinery, including Mediator, leading to enhanced activation of the IGF1R promoter and accumulation of additional IGF1R transcripts. (D) Bloodlinc potentiates the terminal erythropoiesis gene program. RNA FISH image shows nuclear diffusion of Bloodlinc molecules. Transcript models from mouse and human are shown at bottom. Bloodlinc diffuses from a super-enhancer domain to trans-loci encoding terminal erythropoiesis modulators, and binds HNRNPU as well as transcription coactivators/corepressors, mediating activation or repression of its targets.
Subsequent deposition of repressive chromatin marks involves Xist binding to HNRNPK through its B-F repeat domain,41,58-60 which leads to recruitment of the PRC1/2 silencing complexes through unknown mechanisms. As Xist spreads to physically proximal loci, it repositions them into a compact compartment, although it is unclear if this relocation underlies or is a consequence of their silencing. The compaction process is thought to involve direct binding of Xist to LBR, a nuclear lamina anchoring factor.58,60,61 Xist’s varied interactions involve independent protein interaction domains, making it a paradigm for lncRNAs that act as modular scaffolds to spatially integrate and coordinate the functions of diverse multiprotein complexes.
lncRNAs also recruit regulatory proteins in lineage-committed blood cells. We identified lincRNA-EPS, which diffuses throughout the nucleus to repress loci encoding common mediators of apoptotic and inflammatory signaling in red cells and macrophages (Figure 2B).62,63 These include the NF-κB subunit NFKB1 and PYCARD/ASC, which mediates assembly of inflammatory and apoptotic signaling complexes via caspase activation. Silencing of these mediators by lincRNA-EPS is vital for cell survival during terminal erythropoiesis, such that ectopically expressed lincRNA-EPS can rescue progenitors from apoptosis caused by erythropoietin starvation. In macrophages, silencing of these mediators by lincRNA-EPS restrains uncontrolled inflammatory responses, such that lincRNA-EPS−/− mice show elevated systemic cytokine levels causing higher susceptibility to lethal endotoxic shock.
Mechanistically, lincRNA-EPS binds the promoters of its target genes and promotes nucleosome occupancy near their transcription start site, thus repressing their basal expression. These effects depend on binding HNRNPL via a CANACA motif within a 3′-end 500-nt domain of lincRNA-EPS that is required for its function. How lincRNA-EPS reaches its targets and the molecular roles played by HRNPL (or other partners) in mediating its repressive action remain to be elucidated.
Illustrating a cis mechanism of action, linc-MAF-4, a chromatin-retained lncRNA specifically expressed in the TH1 subset of helper T cells, represses expression of MAF, a TF associated with TH2 cells.64 The linc-MAF-4 locus is brought into contact with the nearby MAF promoter via a long-range chromatin loop, and the linc-MAF-4 transcript binds the chromatin-silencing factors EZH2 and LSD1. Inhibiting linc-MAF-4 reduces chromatin occupancy of EZH2 and LSD1 and attenuates H3K27me3 deposition specifically at the MAF promoter. Although the main mechanisms of MAF repression remain unclear, linc-MAF-4–deficient cells upregulate MAF and preferentially differentiate toward the TH2 phenotype. Interestingly, several other lncRNAs have been found to modulate immune cell differentiation, lifespan, and function by acting as recruiters of transcription/chromatin regulators in cis and trans (Table 1).65-69
Enhancer-associated lncRNAs can also scaffold regulators in cis and trans. LUNAR1 is an lncRNA whose expression is induced by oncogenic NOTCH1 signaling specifically in T-cell acute lymphoblastic leukemia (T-ALL) (Figure 2C).70 LUNAR1 is cis-retained within a DNA loop between its promoter and an intronic IGF1R enhancer and is needed for robust enhancer engagement by the Mediator complex and RNA polymerase II. Accordingly, endogenous but not ectopic LUNAR1 expression triggers high-level IGF1R expression, and LUNAR1-deficient T-ALL cells show a competitive growth disadvantage in vivo because of impaired IGF1 signaling. These effects are absent in myeloid leukemia cells, which rarely involve aberrant NOTCH1 signaling71,72 and consequently lack LUNAR1 expression. Although IGF1R may not be LUNAR1’s sole target, its role in modulating IGF1 signaling in NOTCH1-dependent T-ALL renders it an attractive target for T-ALL–specific therapies.
Recently, we characterized alncRNA-EC7/Bloodlinc, a conserved RNA that diffuses throughout the nucleus (Figure 2D).34,35,73 Bloodlinc is transcribed from an enhancer required for high-level SLC4A1/BAND3 erythroid expression, but is not cis-retained. Instead, it localizes to trans-chromosomal loci encoding key terminal erythropoiesis mediators (eg, HIPK1, GATA2) and is required for their proper modulation. As evidence, expression of these genes is reciprocally regulated upon Bloodlinc depletion or ectopic overexpression. Bloodlinc binds the HNRNPU chromatin attachment factor and the nuclear lamina component LMA, and can bind transcription coactivators (eg, MYBBP1A) and corepressors (eg, HSPA8). This suggests that Bloodlinc scaffolds distinct factors to stimulate or repress target gene expression, although the determinants of whether (and how) it brings repressive or activating factors to its targets remain to be elucidated. Importantly, Bloodlinc is required for terminal red cell maturation and is able to potentiate it because ectopically expressed Bloodlinc can promote erythroblast proliferation and enucleation in the absence of differentiation stimuli.
The lncRNAs described here recruit broadly expressed proteins, including HNRNPU/K/L and PRC1/2, to specific sites within chromatin, thereby imparting spatially defined roles. This illustrates a general principle whereby lncRNAs partner with ubiquitous regulators to direct context-specific gene programs, a common mode of regulation during normal and malignant hematopoiesis.32,33,45 Cell type–specific lncRNAs (eg, LUNAR1, Bloodlinc) exploit such partnerships to exert cell type–specific gene control with exquisite spatiotemporal precision.
lncRNAs can also impart such precision posttranscriptionally by scaffolding factors that mediate RNA processing (eg, RNA unwinding/splicing/packaging/transport), translation, or RNA degradation. For example, the ubiquitously expressed lncRNA MALAT1 binds SR splicing factors to regulate alternative splicing across many active gene loci.39,74 lncRNA recruiters of posttranscriptional modifiers have not been studied in depth in the hematopoietic system, however, partly because of the technical challenges of specifically detecting and characterizing complexes containing mRNA, protein, and lncRNA components.
Many lncRNAs sequester proteins away from their targets
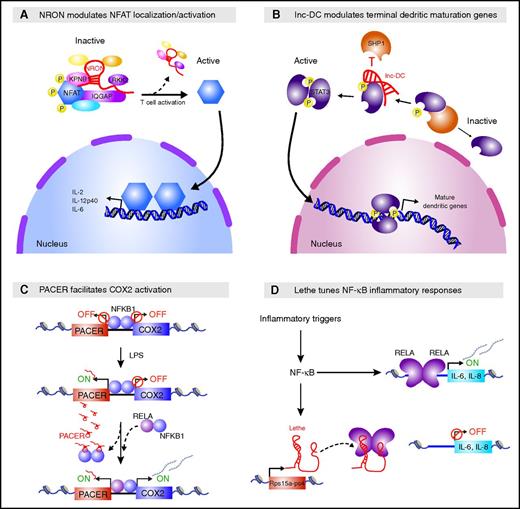
Spatiotemporal gene control can also be exerted by sequestering proteins away from their targets. The lncRNA NRON uses this strategy to tune the activity of NFAT, a calcium-dependent TF critical for T-cell activation (Figure 3A).75-77 NRON sequesters phosphorylated NFAT in the cytoplasm of resting cells by scaffolding it within a complex that includes the calmodulin-binding protein IQGAP1, the nuclear importer KPNB, and the inhibitory kinase LRRK2. Stimulated cells that are NRON- or LRRK2-deficient show higher dephosphorylation and nuclear translocation of NFAT, causing elevated NFAT-dependent cytokine production. Consistent with this phenotype, Lrkk2−/− mice are more susceptible to induced autoimmune colitis (NRON-deficient mice have not been studied). Thus, like lincRNA-EPS in macrophages, NRON restricts inappropriate and potentially harmful inflammatory responses.
Many lncRNAs sequester proteins from their targets. (A) NRON sequesters phosphorylated NFAT in the cytoplasm. NRON scaffolds NFAT within a complex that includes the calmodulin-binding protein IQGAP1, the nuclear importer KPNB1, and the inhibitory kinase LRRK2, keeping NFAT in a phosphorylated inactive state. T-cell activation leads to increased Ca2+ levels, causing disassembly of this complex and NFAT dephosphorylation, which in turn favors NFAT nuclear import and subsequent activation of cytokine expression. (B) lnc-DC promotes the terminal dendritic maturation gene program. lnc-DC binds the C terminus of STAT3 and sequesters it away from the phosphatase SHP1, preventing its dephosphorylation and favoring nuclear import of phosphorylated STAT3 and subsequent activation of the terminal DC differentiation gene program. (C) PACER promotes COX2 activation in cis. PACER is induced as part of the inflammatory response in response to lipopolysaccharide (LPS) stimulation. PACER binds to and competes with the repressive NF-κB1 homodimer away from the adjacent COX2 promoter, favoring binding of the activating RELA/NF-κB1 heterodimer to the promoter and subsequent COX2 activation. (D) Lethe tunes inflammatory responses in trans. Lethe, a chromatin-retained lncRNA, is transcribed from the Rps15a-ps4 pseudogene and acts as an RNA decoy that titrates activating RELA complexes away from target gene promoters, restraining cytokine activation downstream of inflammatory triggers.
Many lncRNAs sequester proteins from their targets. (A) NRON sequesters phosphorylated NFAT in the cytoplasm. NRON scaffolds NFAT within a complex that includes the calmodulin-binding protein IQGAP1, the nuclear importer KPNB1, and the inhibitory kinase LRRK2, keeping NFAT in a phosphorylated inactive state. T-cell activation leads to increased Ca2+ levels, causing disassembly of this complex and NFAT dephosphorylation, which in turn favors NFAT nuclear import and subsequent activation of cytokine expression. (B) lnc-DC promotes the terminal dendritic maturation gene program. lnc-DC binds the C terminus of STAT3 and sequesters it away from the phosphatase SHP1, preventing its dephosphorylation and favoring nuclear import of phosphorylated STAT3 and subsequent activation of the terminal DC differentiation gene program. (C) PACER promotes COX2 activation in cis. PACER is induced as part of the inflammatory response in response to lipopolysaccharide (LPS) stimulation. PACER binds to and competes with the repressive NF-κB1 homodimer away from the adjacent COX2 promoter, favoring binding of the activating RELA/NF-κB1 heterodimer to the promoter and subsequent COX2 activation. (D) Lethe tunes inflammatory responses in trans. Lethe, a chromatin-retained lncRNA, is transcribed from the Rps15a-ps4 pseudogene and acts as an RNA decoy that titrates activating RELA complexes away from target gene promoters, restraining cytokine activation downstream of inflammatory triggers.
A similar mechanism is employed by human lnc-DC to modulate activity of STAT3, a TF required for DC differentiation (Figure 3B).36 lnc-DC binds the C terminus of STAT3 in the cytoplasm and acts as a decoy to prevent STAT3 from binding the SHP1 phosphatase and subsequently undergoing dephosphorylation. As such, lnc-DC keeps STAT3 in an active state that favors its nuclear import. Accordingly, lnc-DC deficiency causes cytoplasmic retention of inactive (dephosphorylated) STAT3, which compromises the terminal DC differentiation gene program. This impairs production of mature DCs capable of antigen uptake and allogeneic T-cell stimulation in vitro. RNA interference-mediated knockdown of mouse lnc-DC similarly impairs DC differentiation in vivo; however, the mouse lnc-DC ortholog (and every nonhominid primate ortholog examined) encodes a small secreted protein,78 obscuring interpretation of this phenotype.
Another lncRNA, GAS5, modulates T-cell proliferation and survival by titrating the glucocorticoid receptor (GR) away from its targets.3 Primary GAS5 transcripts host multiple intronic small nucleolar RNAs, whereas spliced GAS5 is rapidly degraded in the cytoplasm by the NMD machinery. Accordingly, inhibition of translation by growth arrest or by chemical inhibitors (eg, cycloheximide, rapamycin) is accompanied by GAS5 accumulation. In T cells, GAS5 is required for growth arrest and subsequent apoptosis and for the inhibitory effects of rapamycin. These functions are mediated by GAS5 binding to the DNA-binding domain of ligand-activated GR in the cytoplasm, via a 3′-end motif whose secondary structure acts as a decoy steroid receptor response element.79 This RNA motif can be traced to the last common ancestor of tarsiers and simians,80 illustrating a conserved lncRNA structure–function relationship. GR-bound GAS5 migrates into the nucleus, where it competes with DNA glucocorticoid response elements for binding to the GR. GAS5 thus attenuates GR-mediated induction of target genes, including antiapoptotic ones (eg, CIAP2), thereby sensitizing cells to apoptosis. Intriguingly, GAS5 genetic lesions and GAS5 downregulation have been linked to the pathogenesis of various leukemia and lymphoma types, casting it as a potential lymphoid tumor suppressor. This warrants further probing of the phenotypes of GAS5 modulation under physiologically informative conditions.
lncRNAs can also sequester regulators from their nuclear targets in cis. PACER, an lncRNA induced by lipopolysaccharide stimulation in human macrophages, promotes expression of the neighboring PTGS2/COX2 gene, encoding an inflammatory response modulator (Figure 3C).81 PACER binds to and titrates the repressive NF-κB1 homodimer away from the COX2 promoter, thereby facilitating binding of the activating RELA/NF-κB1 heterodimer and subsequent formation of transcription preinitiation complexes. COX2 deregulation has been linked to the pathogenesis of various cancers,82 making PACER an attractive target for therapeutic modulation of COX2 transcription, although the physiological viability of such an approach remains unclear.
Another class of lncRNAs, originating from pseudogenes, may also act as decoys. Lethe, a chromatin-retained lncRNA transcribed from the Rps15a-ps4 pseudogene, tunes NF-κB signaling downstream of inflammatory triggers (Figure 3D).83 Lethe binds to and titrates the activating RELA subunit away from the promoters of target genes, including those encoding NFKBIA and the interleukin-6/interleukin-8 cytokines, thereby restraining the NF-κB–dependent inflammatory response. Accordingly, ectopically expressed Lethe diminishes NF-κB–dependent gene activity in a dose-dependent manner. Lethe itself is NF-κB–inducible, making it part of a negative feedback control mechanism for NF-κB signaling. Intriguingly, Lethe exhibits age-associated suppression, suggesting potential physiological roles in elevated NF-κB–dependent inflammatory activity with aging.
lncRNAs may also titrate posttranscriptional regulators away from their RNA targets, although such mechanisms are less well understood. For example, the FAS-AS1 lncRNA sequesters RBM5 from the FAS receptor pre-mRNA and thus prevents its alternative splicing.84 RBM5-mediated skipping of Fas exon 6, which encodes the FAS trans-membrane domain, generates a soluble FAS receptor that binds to FAS ligand. Upregulation of this decoy receptor isoform is one of the strategies used by malignant B-cell lymphomas to evade FAS-mediated apoptosis, a common chemotherapy regimen. Accordingly, soluble FAS serum levels are associated with poor prognosis of non-Hodgkin lymphomas.85 Both endogenous and ectopic FAS-AS1 can modulate FAS signaling by suppressing soluble FAS production, making FAS-AS1 an attractive therapeutic target to combat malignant lymphoma chemoresistance. This warrants further study of potential FAS-AS1 RNA targets, interacting proteins, and physiological phenotypes.
Other mechanisms of lncRNA function
Besides regulating traffic between proteins and their targets, lncRNAs may also serve as allosteric/structural/catalytic components of ribonucleoprotein complexes (eg, ribosomal and RNase P/MRP RNA). Moreover, lncRNAs that are transcribed antisense to mRNAs have a natural capacity to act as endogenous antisense oligonucleotides and potentially modulate processing of their cognate mRNA. These have been termed “natural antisense transcripts,” although many are rapidly degraded in the nucleus and lack RNA-based functions (reviewed in Pelechano and Steinmetz86 ). In hematopoietic cells, the antisense lncRNAs PU.1-AS and AS-RMB15 have been shown to inhibit and promote translation of the PU.1 and RBM15 regulators, respectively.87,88 The molecular pathways by which lncRNAs such as these impact translation are poorly understood, however. Base-pairing to lncRNAs may also modulate other aspects of mRNA processing, such as degradation,89,90 but such action modes remain unexplored in hematopoietic cells.
lncRNAs can also base pair to other types of regulatory RNAs such as microRNAs, potentially titrating microRNA effector complexes away from their mRNA targets.6,31 lnc-MC, for example, is thought to sequester miR199a-5p and thus promote human myeloid differentiation.91 miR199a-5p inactivates the mRNA encoding ACVR1B, an important regulator of monocyte/macrophage differentiation. Ectopically expressed lnc-MC binds to and sequesters miR199a-5p, which alleviates ACVR1B repression and leads to activation of transforming growth factor-β signaling, thereby promoting monocytopoiesis. The molecular pathways by which lncRNAs such as lnc-MC compete with other transcripts to impact microRNA targeting are poorly understood, however. Experiments focusing on microRNA sponging, as exemplified by lnc-MC, tend to overexpress RNA competitors beyond physiological levels, which may not recapitulate natural settings.92-95
In sum, lncRNAs can interact with proteins or with nucleic acids to modulate their processing, localization, or function, which enables them to regulate cellular development and homeostasis at multiple levels. Because of their flexible and dynamic action modes, lncRNAs can integrate extracellular inputs and direct spatially and temporally coordinated gene responses, allowing cells to rapidly adapt to changing developmental and environmental signals. Indeed, the examples here illustrate how lncRNAs mediate key hematopoietic pathways under developmental-, aging-, and disease-specific contexts.
Probing lncRNA mechanisms: lessons learned
An expanding toolbox of molecular assays is rapidly becoming available to query lncRNA molecular mechanisms (see Goff and Rinn96 ; supplemental Text 3). Here, we discuss recently learned lessons in the refinement of strategies to probe lncRNA mechanisms.
Ruling out peptide-based mechanisms
Whether protein-coding capacity can be ruled out from putative lncRNAs has been amply debated.97-99 Proteomic studies find little support for productive lncRNA translation,100-103 yet sequencing of ribosome-protected RNA can yield reads within lncRNAs, suggesting their translation.104-107 Using empirical criteria based on the ribosome-protected RNA enrichment and delineation features characteristic of known open reading frames (ORFs), we found evidence of active translation for just 3% of erythroid lncRNAs,108 consistent with upper-boundary estimates of lncRNA misidentification.101,109 Nonetheless, misidentified lncRNAs included lincRNA-EC2/Redrum, which is critical for red cell maturation,34,35 calling into question whether it functions as a noncoding RNA or through an encoded peptide.
Whether putative lncRNAs produce peptides may only matter if they affect function. Nuclear lncRNAs that occasionally reach the cytoplasm may undergo nonfunctional translation, whereas cytoplasmic lncRNAs might use upstream ORFs as decoys to prevent ribosomes from interfering with functional RNA domains.5 Further, some lncRNAs engage ribosomes as part of their regulatory mechanisms, and translation-dependent NMD is the normal decay pathway for certain lncRNAs.22,87,110-112 Given increasingly recognized functions for small peptides,98 however, functional interrogation is ultimately needed to prove noncoding action. For example, disrupting all 4 putative ORFs within lincRNA-EPS did not alter its antiapoptotic function,62 demonstrating its noncoding action. Of note, bifunctional genes exist that produce isoforms acting as lncRNAs as well as isoforms encoding functional peptides,113,114 but such functions are separable and can still be mechanistically dissected.
Dissecting cis vs trans RNA-based mechanisms
lncRNAs retained at their locus and that affect expression of nearby genes are typically thought to act locally. We initially interpreted Bloodlinc to act in this way because it is required for induction of the nearby Band3 gene and forms RNA foci upon induction in early- to mid-stage differentiating erythroblasts.34 However, studying Bloodlinc in late-stage differentiating erythroblasts revealed a diffuse RNA,35 unbiased interrogation uncovered >200 target loci (including Band3), and ectopic expression demonstrated trans-functions.73 Similar realizations have emerged as well-known lncRNAs are studied in increasing detail.115,116 For example, Evf2, initially found to regulate gene expression in cis (via antisense regulation),117 is now thought to additionally prevent enhancer methylation in trans (by scaffolding regulators).115,118 Thus, dissecting cis vs trans function demands investigating RNA diffusion patterns under informative physiological contexts, querying for potential targets genome-wide, and scrutinizing broad cellular functions. lncRNAs are mechanistically diverse; therefore, diverse consequences of lncRNA perturbation must be queried both locally and globally to thoroughly dissect their functional modalities.
Dissecting functionally meaningful RNA–protein partnerships
The mechanisms of virtually all lncRNAs involve binding proteins; however, nonspecific binding of many lncRNAs to certain proteins, including chromatin and DNA-binding factors, has been increasingly reported.119-121 Nonspecific RNA–protein interactions are a concern in native purification assays because of nonphysiological RNA–protein binding in solution, especially for abundant proteins (eg, hnRNPs) and abundant RNAs. Crosslinking-based purifications can demonstrate a specific RNA–protein interaction and thus alleviate this concern, but are limited by the efficiency of crosslinking and cannot be applied to primary tissues. RNA-centric purifications represent and improvement over protein-centric assays for unbiased detection of lncRNA–protein interactions, but they are limited by the abundance of the target lncRNA. Because of these concerns, method-dependent controls must be carefully designed, such as noncrosslinked samples for crosslinking-based purifications, and sensitivity and specificity controls for hybridization-based RNA capture methods (supplemental Text 3). Regardless of the method used to identify them, putative lncRNA–protein interactions must ultimately be verified via multiple complementary approaches. Even when lncRNA–protein interactions can be verified, however, these may not be informative about the lncRNA’s specific functions. Many lncRNAs bind ubiquitous RNA splicing, unwinding, packaging, transport, and decay factors as part of their normal processing. Such proteins inevitably bear phenotypes when disturbed because of their broad functions. Hence, discerning whether verified lncRNA–protein interactions reflect general RNA processing or influence the specific functions of the mature lncRNA is key to unraveling its mechanisms. Disrupting specific lncRNA–protein interactions, ideally by mapping lncRNA interaction domains and probing the functional impact of perturbing them, provides a powerful tool to answer this question.
Dissecting in vivo lncRNA functions
A growing number of in vivo lncRNA studies have reported discrepancies with phenotypes seen in cultured cells.122-124 For example, lincRNA-EPS−/− mice show unrestrained inflammatory responses but have normal erythrocyte and hemoglobin levels, contrasting with a critical requirement for lincRNA-EPS in the survival of terminally differentiating erythroblasts ex vivo. Such discrepancies may reflect functional redundancies or compensatory physiological responses that mask lncRNA function, similar to effects of deleting many protein-coding genes. Alternatively, they may reflect roles specific to ex vivo/in vitro cellular contexts that are absent in vivo. Thus, functional assays in the native physiological contexts are ultimately needed to elucidate physiologically relevant lncRNA functions.
Future prospects
Judging by the recent surge in RNA-centric tools for unbiased interrogation of lncRNA targets, partners, structure, and functions (reviewed in Goff and Rinn96 ), we expect mechanistic lncRNA studies to flourish in the coming years. As with the study of proteins, however, we expect no single study to decisively unravel the precise mechanisms of a given lncRNA. Rather, we suspect that multiple lines of evidence from diverse approaches, including RNA-based perturbations, rescue assays, deletion-mapping, and structure-function studies, will be needed to clarify lncRNA mechanisms. Although the road ahead might be challenging, there is great promise of finding answers to salient questions about how lncRNAs function. For example, what features determine whether lncRNAs diffuse to trans-loci or remain cis-tethered to their transcription sites? How does local chromatin state and conformation influence whether lncRNAs activate or repress their genomic targets? And how is specificity of interactions with chromatin/RNA/protein targets achieved and modulated across cellular and developmental contexts?
With accumulating knowledge from diverse lncRNA examples, common modes of lncRNA function should begin to become apparent. It will be especially interesting to uncover common RNA sequences, structures, and domains that allow for predictive models of lncRNA function, just as identification of protein domains does for proteins. These should shed light onto which molecular features dictate lncRNA localization, interaction capabilities, and mechanistic modalities. Ultimately, answering these questions may illuminate why organisms evolved ribonucleoprotein-mediated regulation, instead of solely protein-based regulation, to enact developmental programming. At the same time, it might lead to a better understanding of how lncRNAs contribute to the aging, malfunction, and malignant transformation of blood cells, potentially opening new avenues for research and therapeutic manipulation of these processes.
The online version of this article contains a data supplement.
Acknowledgments
The authors apologize to colleagues whose work could not be cited or discussed owing to space limitations, and thank Marko Knoll for critical comments on the manuscript and Tom DiCesare for help with illustrations. J.R.A.-D. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation.
This research was supported by grants from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (DK068348), and NIH National Heart Lung, and Blood Institute (5P01 HL066105) (H.F.L.).
Authorship
Contribution: J.R.A.-D. and H.F.L. designed and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan R. Alvarez-Dominguez, Department of Stem Cell and Regenerative Biology, Harvard Stem Cell Institute, Harvard University, Cambridge, MA 02138; e-mail: juanralvarez@fas.harvard.edu; and Harvey F. Lodish, Whitehead Institute for Biomedical Research, 9 Cambridge Ctr, Cambridge, MA 02142; e-mail: lodish@wi.mit.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal