Abstract
Introduction: Multiple myeloma plasma cells (MM-PCs) home to and dynamically interact with the bone marrow (BM) microenvironment (BM-ME). Of the different factors secreted by the BM-ME, NF-κB-induced cytokines including IL-6 play a central role in MM-PC survival and resistance to treatments. Previously published data have shown that in the BM of MM patients the presence of CD163+ tumor associated macrophages (TAM) correlates with IL-6 production. The canonical NF-κB pathway is induced by most physiological stimuli to produce low levels of inflammatory cytokines, through the activation of the IκB kinase complex (IKKβ, IKKα, and IKKγ). This pathway seems constitutively activated in TAM, where instead the activation of the non-canonical pathway seems to play a critical role in the anti-cancer phenotype of BM macrophages (BM-Mϕ).
Extracellular vesicles (EV) are membrane-covered cell fragments released by all cell types, and recently they have been shown to play a pivotal role in creating a tumor supportive BM-ME. Our group was the first to show that MM cells produce EV (Harshman et.al. 2013). Previous studies have shown that miR- 16 encapsulated inEV(EV-miR- 16) down-modulates IL-6 production in the BM-ME, and we showed that higher levels of circulating miR- 16 is a prognostic factor that correlates with better survival in newly diagnosed MM patients treated with proteasome inhibitor (PI) based therapy (Rocci et al. 2013). Here, we investigated whether MM derived EV can play a pivotal role in supporting monocyte to TAM differentiation and defined the molecular mechanisms of IL-6 modulation by miR- 16 .
Methods: Monocyte to TAM differentiation was evaluated by flow cytometry analysis, light microscopy, q-RT-PCR and phagocytosis assays. EV were isolated via a serial centrifugation method. EV RNA content was analyzed by NanoString. EV isolated from producing cells (0.5x106cells/ml) were used to differentiate 1 monocyte (4:1 effector:target ratio). Cytokine arrays in the supernatant of BM-Mϕ and in the BM-Mϕ depleted fractions were performed. Cloning, Western blot and RT-PCR were used to confirm IKK complex targeting by miR- 16 . Single cell flow and NF-kB activation assays were conducted.
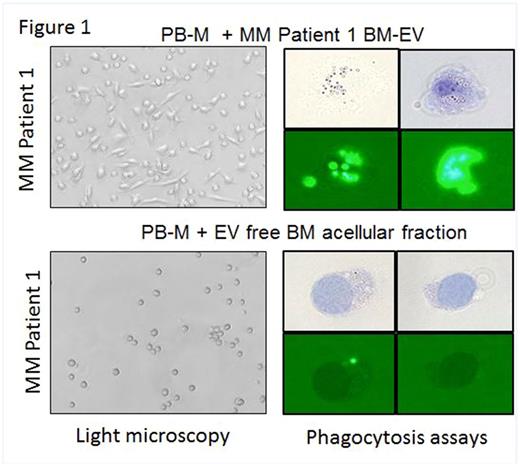
Results: Our in vitro data show that EV isolated from the supernatant of MM cell lines (MM-EV) can promotemonocyte differentiation to CD163+ TAM. Mϕ differentiation was also observed when peripheral blood monocytes (PB-M) obtained from both MM and healthy donors were treated with EV isolated from the BM acellular fraction of MM patients (n=4). Limited differentiation was instead observed when the acellular fraction was EV-depleted (Fig. 1). Our data show that PB-M differentiation was partially reversed by the addition of an EV-miR- 16 mimic. We found that miR- 16 is dramatically down-regulated when PB-M is induced by macrophage colony stimulating factor to differentiate to TAM, and it is also down-regulated in primary BM-Mϕ as compared to PB-M of the same patients and to the BM-Mϕ of 3 age matched healthy donors. We learned that BM-Mϕ isolated from MM patients are the main source of NF - κB-induced cytokines including IL-6 and IL-10 under unstimulated conditions as well as IL-6, IL-8, IL-10, TNF-α and VEGF under Toll-like receptor stimulated conditions, as the Mϕ-depleted BM cellular fraction had significantly low levels. We also found that miR -16 ectopic expression in MM cells and in a human monocytic cell line downregulates both IKKβ/IKKα protein and mRNA levels, with subsequent downregulation of NF-κB activation signaling and decreased expression of inflammatory cytokines. Further, cell growth and apoptotic assays show that the attenuation of PI-based anti-MM activity by TAM is partially reversed by ectopic miR- 16 in both TAM and MM-PCs.
Conclusions: MM-EV support monocyte differentiation to TAM, causing miR- 16 downregulation and an increase in NF-κB inflammatory signaling. We also show that this effect can be impaired by an ectopic miR- 16 mimic. Since constitutive activation of NF-κB decreases MM cells dependency on extrinsic growth factors, at the onset of the disease miR- 16 downregulation in MM-PCs can support NF-kB intrinsic activation and increased fitness of MM cells. Because NF-kB activation in MM-PCs counterbalances the anti-MM activity of PI, it is not surprising that miR- 16 expression can potentiate the killing effect of PI. We are exploring these hypotheses through use of miR -16 knock out mouse models.
Hofmeister: Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Karyopharm: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Roche: Research Funding; Thrassos: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding. Krishnan: Takeda: Speakers Bureau; Onyx: Speakers Bureau; Sutro: Consultancy; Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Equity Ownership, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal