Abstract
Introduction. Acute myeloid leukemia (AML) encompasses heterogeneous entities, but global outcome remains poor. Among genetic AML subgroups, complex karyotypes represent one of the most difficult-to-treat. Genomic alterations arising from recent approaches in molecular genetics have allowed describing new subsets with potential therapeutic implications. Besides, the latter AML classification ("2017 European LeukemiaNet (ELN) risk stratification") integrates both molecular alterations and conventional cytogenetics. High-resolution single-nucleotide polymorphism array (SNP-Array) identifies copy number alterations (CNA) and copy-neutral losses of heterozygosity (CN-LOH). In the randomized ALFA-0702 study (NCT 00932412), we have recently reported the effects of a CLARA combination (clofarabine + intermediate-dose cytarabine) as compared to standard high-dose cytarabine (HDAC) regimen as consolidation chemotherapy in younger adults with AML. Here, we aimed at evaluating the effects of the CLARA regimen according to patient subsets defined by SNP-array abnormalities.
Methods. A total of 221 patients aged 18-59 years old with intermediate- or adverse-risk de novo AML in first complete remission (CR/CRp) after a common intensive induction course were randomized in the ALFA-0702 trial (Thomas et al. JCO 2017). The primary endpoint was relapse-free survival (RFS). Bone marrow or peripheral blood samples at diagnosis were analyzed by Cytoscan HD array (Affymetrix) according to the manufacturer's instructions. Data were screened according to the ChAS 3.1 algorithm, with stringent filter retaining CNA larger than 20 kb - 20 markers, telomeric CN-LOH > 3Mb and interstitial CN-LOH > 10Mb.
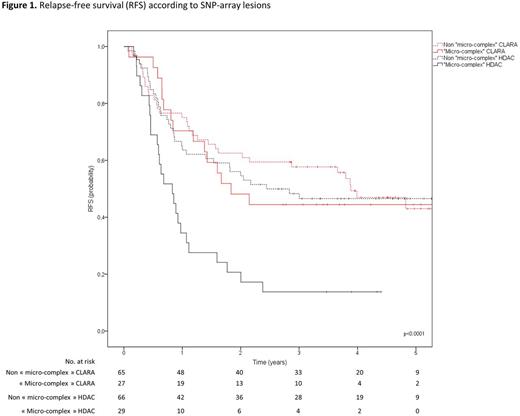
Results. SNP-array was successfully performed in 187 out of the 221 randomized patients (92 CLARA arm, 95 HDAC arm). Twenty-three patients could not be analyzed in SNP-array for the following reasons: DNA sample was not available (15 patients) or in insufficient quantity (8 patients). SNP-array analysis failed in 11 patients. Median number of genomic SNP-Array abnormalities reached 2 per patient (range, 0-46) and did not significantly differ between both randomization arms (p=0.41). CNA accounted for the most common SNP-Array abnormalities (Loss=54%, Gain=38%, vs. CN-LOH=8%). Conventional cytogenetic analysis showed a complex karyotype (defined by at least 3 chromosomal abnormalities) in 37 patients (11 CLARA, 16 HDAC). In such patients, CLARA consolidation improved RFS (median RFS, 22 vs. 8 months; p=0.027). Interestingly, a similar impact was observed in the 56 patients (27 CLARA, 29 HDAC) with micro-complex karyotypes defined by 4 or more SNP-Array abnormalities. Notably, 7 patients with complex karyotype did not display micro-complex karyotype because of low bone marrow/blood blast infiltration and/or balanced alterations. In patients with micro-complex karyotypes, median RFS was 22 months in the CLARA arm compared to 10 months in the HDAC arm (p<.0001). The rate of patients who received allogeneic stem cell transplantation in first CR did not differ between the two arms (p=0.99). Importantly, CLARA consolidation tended to overcome the poor prognostic impact of patients with micro-complex karyotype. Indeed, patients with such lesions had similar 4-years RFS than other patients in the CLARA arm (44% vs. 47%) while it remained a poor prognosis factor in the HDAC arm (14% vs. 47%; Figure 1).
Conclusion. As compared to conventional cytogenetics, SNP-array represents a high-resolution approach to better characterize molecular profile of AML patients by pointing out cryptic molecular abnormalities. Our results suggest that clofarabine-based consolidation benefits both patients with complex karyotypes and micro-complex karyotypes defined by 4 and more SNP-Array abnormalities. Therefore by delineating micro-complex karyotypes in SNP-array, we have defined a new subset of AML patients that could potentially benefit from a clofarabine-based consolidation regimen (21 additional patients in comparison with complex karyotypes). SNP-array could thus help to improve AML management by refining adverse patient subgroups that could potentially benefit from new alternative consolidation regimen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal