Abstract
The hemostatic system plays pivotal roles in injury repair, innate immunity, and adaptation to inflammatory challenges. We review the evidence that these vascular-protective mechanisms have nontraditional roles in hematopoietic stem cell (HSC) maintenance in their physiological bone marrow (BM) niches at steady-state and under stress. Expression of coagulation factors and the extrinsic coagulation initiator tissue factor by osteoblasts, tissue-resident macrophages, and megakaryocytes suggests that endosteal and vascular HSC niches are functionally regulated by extravascular coagulation. The anticoagulant endothelial protein C receptor (EPCR; Procr) is highly expressed by primitive BM HSCs and endothelial cells. EPCR is associated with its major ligand, activated protein C (aPC), in proximity to thrombomodulin-positive blood vessels, enforcing HSC integrin α4 adhesion and chemotherapy resistance in the context of CXCL12-CXCR4 niche retention signals. Protease-activated receptor 1–biased signaling by EPCR-aPC also maintains HSC retention, whereas thrombin signaling activates HSC motility and BM egress. Furthermore, HSC mobilization under stress is enhanced by the fibrinolytic and complement cascades that target HSCs and their BM niches. In addition, coagulation, fibrinolysis, and HSC-derived progeny, including megakaryocytes, synergize to reestablish functional perivascular HSC niches during BM stress. Therapeutic restoration of the anticoagulant pathway has preclinical efficacy in reversing BM failure following radiation injury, but questions remain about how antithrombotic therapy influences extravascular coagulation in HSC maintenance and hematopoiesis.
Introduction
Hemostasis is achieved by the coordinated activation of coagulation and platelets and limits blood loss from damaged or stressed vessels. Hemostatic plug formation by platelets and the tissue factor (TF) coagulation pathway are central to normal hemostasis.1 The cell surface receptor TF, in complex with its protease ligand, coagulation factor FVIIa, also functions outside of the vascular compartment. In the context of leaked or locally synthesized coagulation factors, TF-FVIIa can trigger extravascular coagulation by activating key coagulation factors (FIX and FX) and cofactors (FVIII and FV).2 Similarly, TF pathway inhibitor is synthesized by megakaryocytes and vessel wall cells and, thereby, is positioned beyond the vascular space to regulate TF or the FVa-FXa prothrombinase that generates the central coagulation protease thrombin (FIIa).3
In addition to blood-coagulation inhibitors, thrombin generation in the circulation is effectively controlled by the anticoagulant protein C (PC) pathway, preventing thrombosis.4 Generated thrombin is captured by endothelial cell–expressed thrombomodulin (TM; CD141), preventing thrombin’s coagulant function. Instead, the TM–thrombin complex activates PC bound to the endothelial PC receptor (EPCR; CD201), as well as the carboxypeptidase thrombin-activatable fibrinolysis inhibitor (TAFI). Activated PC (aPC) proteolytically inactivates coagulation FVa and FVIIIa, limiting coagulation amplification, whereas TAFI modifies fibrin, thrombin-cleaved osteopontin, and complement-activation fragments C3a and C5a, counteracting fibrinolysis and inflammation.5 In addition, the fibrinolytic system prevents excessive fibrin deposition and facilitates restoration of tissue integrity after injury. Expanding evidence indicates that these vascular-protective and -repair systems are physiological regulators of hematopoietic stem and progenitor cells (HSPCs) and their niches in the bone marrow (BM).
The PC pathway has important functions in vascular homeostasis by promoting vascular barrier integrity and limiting endothelial inflammation.4 In vascular protection, aPC bound to EPCR activates the G protein–coupled protease-activated receptor 1 (PAR1) and counteracts proapoptotic and proinflammatory signaling by various agonists. Alternative cleavages of PAR1 by coagulation proteases, including thrombin and FXa, at position PAR1-Arg41 vs by the anticoagulant aPC at PAR1-Arg46 results in the generation of unique tethered ligands that govern distinct downstream coupling to G proteins and β-arrestins, with resulting protease-selective PAR1 responses in endothelial cells. Homeostatic, cytoprotective, and immune-regulatory signaling of aPC in other cell types can involve alternative coreceptors (ie, LRP8 or leukocyte integrin CD11b/CD18), as well as proteolytic cleavage of PAR3 that can cross-activate PAR1 and PAR2.6 Conversely, EPCR serves as the receptor for aPC, as well as for FXa7 and FVIIa.8 In addition, EPCR is a cell surface marker for a variety of tissue-specific and cancer stem cells,9,10 as well as for hematopoietic stem cells (HSCs).11 Here, we review the evidence that EPCR functionally contributes to maintenance and retention of HSPCs and discuss how hemostatic pathways and their proteolytic signaling contribute to the steady-state regulation of stem cells in their extravascular niches.
HSPCs are activated in response to injury and infection to meet increased demand for blood cell production. In the response to tissue injury, the coagulation system and platelets broadly interact with other innate host defense pathways and the immune system. For example, coagulation and complement activation are interdependent and reciprocally amplified12,13 and influence diverse blood and immune cell populations. PAR signaling by coagulation and other proteases is integrated into antimicrobial host defense and directly linked to Toll-like receptor (TLR) signaling.14 Although intravascular endothelial cell aPC-EPCR signaling rebalances thrombin-dependent PAR1 signaling, aPC counteracts signaling of more upstream coagulation proteases in myeloid cells relevant for extravascular coagulation. In this context, EPCR supports PAR2 activation by TF-FVIIa–generated FXa7 and, thereby, enables specifically TLR4 induction of interferon-regulated genes.15 In turn, the critical interaction of FXa with EPCR can be interrupted by EPCR occupancy through aPC assembled in the anticoagulant FV–protein S complex.16 Therefore, alternative ligands for EPCR may contribute to regulation of HSCs when BM niches are altered by the local activation of immune cells or through remote cues from the periphery. This review will also discuss how the hemostatic system is intertwined with responses and protection of hematopoiesis under stress conditions and inflammation.
HSC regulation in BM stem cell niches
HSCs replenish the hematopoietic system under homeostatic conditions
Quiescent, long-term repopulating HSCs are at the apex of all mature blood cells and maintain a primitive and multipotent pool throughout life by their self-renewal and asymmetrical cell-division potential. In the steady-state, HSC-derived progeny endowed with rapid proliferation capacity in response to stimuli from the periphery replace the loss of activated, consumed, or aged blood cells.17-20 Multilineage repopulation capacity in serial-transplantation experiments is the gold standard to define the most primitive self-renewing HSCs and surface marker patterns (eg, LSK-SLAM [Lin− Sca-1+ cKit+ (CD117) CD48− (SLAMF2) CD150+ (SLAMF1) CD34−] have been established to highly enrich these populations).18 Hematopoiesis is maintained by a stepwise expansion of progenitors in a tree-like hierarchy of branches through common erythrocyte/myeloid and lymphoid progenitors generating oligo-, bi-, and unipotent progenitors and mature blood cells.21 However, recent single-cell transcript profiling indicates that differentiation in hematopoiesis likely originates from a continuum of lineage-biased HSPCs.22,23
In addition, HSC heterogeneity is consistent with an early differentiation bias toward distinct hematopoietic lineages. The platelet integrin α2b (CD41) identifies HSCs with a myeloid-differentiation potential,24 whereas HSCs expressing von Willebrand factor (VWF) preferentially differentiate into megakaryocytes and myeloid cells.25,26 It has also become clear that progenitor populations do not follow a strict hierarchy of differentiation, because common lymphoid progenitors still possess a significant potential for myeloid progeny under inflammatory conditions.27 In addition, whereas steady-state hematopoiesis can be largely maintained with a markedly reduced pool of quiescent HSCs, experimental myeloablative stress uncovers the crucial roles of HSCs in hematopoiesis.20 Thus, HSC maintenance and retention in BM niches are particularly relevant under a variety of stress conditions that exhaust the pools of rapidly proliferating progenitor populations and require chemotherapy-resistant quiescent BM-retained HSCs for prevention of hematopoietic failure.
HSCs are maintained in specific BM niches under steady-state
HSCs are retained in their BM niche through a combination of adhesive and secreted factors, maintaining HSC quiescence and preventing DNA damage and exhaustion.28 The HSC pool throughout the body is replenished by constitutive recycling and circadian mobilization that are regulated by the sympathetic nervous system.29 HSCs are found in the BM close to endothelial cells forming the sinusoidal network of the BM cavity and in proximity to trabecular bone (endosteal) that is also highly vascularized by arterioles innervated by sympathetic nerves (Figure 1). Although these microenvironments are primarily separated by distance, rather than physical barriers, they provide distinct and partially overlapping extracellular signals that impact HSC responses during steady-state and stress.
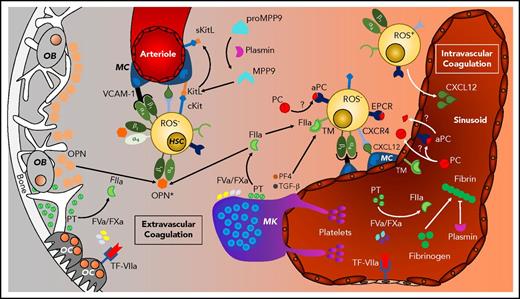
Extravascular coagulation in HSC niche regulation. HSC niches are found in osteoblast (OB)- and osteoclast (OC)-regulated endosteal regions vascularized by arterioles and in megakaryocyte (MK)-rich sinusoidal regions. HSCs are maintained by mesenchymal cell (MC)-derived VCAM, KitL (SCF), CXCL12, and matrix factors (osteopontin; OPN) that are regulated by coagulation and fibrinolysis.
Extravascular coagulation in HSC niche regulation. HSC niches are found in osteoblast (OB)- and osteoclast (OC)-regulated endosteal regions vascularized by arterioles and in megakaryocyte (MK)-rich sinusoidal regions. HSCs are maintained by mesenchymal cell (MC)-derived VCAM, KitL (SCF), CXCL12, and matrix factors (osteopontin; OPN) that are regulated by coagulation and fibrinolysis.
Bone undergoes a constant remodeling by the concerted action of bone-forming osteoblasts and bone-degrading osteoclasts.30 Osteoblasts produce osteopontin and thrombopoietin that render HSC quiescent.31,32 Prothrombin is detectable in the bone matrix,33 and RANKL- differentiated osteoclasts, like macrophages,9,34 produce all components of the TF pathway and generate an active FVa-FXa prothrombinase complex yielding thrombin.35 It is important to consider that endosteal-derived secreted factors may exert more distant effects on HSC retention in perivascular niches. Thrombin cleavage of osteopontin enables integrin α4β1 (VLA4) adhesion of CXCR4+ (CD184) HSCs that is enforced by the chemokine CXCL12 produced by perivascular stromal cells.36,37 In addition, thrombin-cleaved osteopontin supports integrin α9β1 engagement, preventing HSC senescence.38 Thus, bone remodeling and extravascular coagulation interact to shape the BM matrix environment during homeostasis and mobilization.30
HSC maintenance is supported by endothelial cells and mesenchymal stem cell (MSC)-derived stromal cells, specifically CXCL12-abundant reticular (CAR) cells.39 Nestin+ nerve/glial antigen 2+ CAR cells associated with arterioles support HSC retention through CXCL12-CXCR4 signaling,39,40 and nestin+ leptin receptor+ peri-sinusoidal stromal cell–derived stem cell factor (Kit ligand [KitL]) retains HSCs though cKit signaling.40,41 The fibrinolytic system triggered specifically by tissue plasminogen activator has recently been shown to reinforce the latter system.42 Murine BM CD45− Sca-1+ leptin receptor+ and platelet-derived growth factor receptor (PDGFR)α+ (CD140a) MSCs are expanded by the plasmin–MMP-9–mediated release of KitL from MSCs, stimulating cKit+ endothelial cells to secrete the MSC growth factors PDGF-BB and fibroblast growth factor 2 (FGF2).42 Hence, the hemostatic and fibrinolytic systems not only reshape the extravascular matrix environment, but participate in regulating expansion and, possibly, differentiation of the stromal cell compartment in HSPC BM niches.
Anticoagulant pathway components are found in vascular HSC niches
The endosteum is highly vascularized, with pericyte-covered arteries feeding into arterioles and ultimately into sinusoids. The majority of HSCs are localized in close proximity to arteriolar or sinusoidal endothelial cells and associated stromal cells.43-45 In addition to the mesenchymal cell–supported HSC retention signals, endothelial cells are crucial for restoring BM niches after myeloablative stress, for example,46 and maintain HSCs through notch or cKit signaling.41,47 Arterioles and sinusoidal vessels differ in permeability, which may contribute to the availability of plasma-derived coagulation factors, specifically in the more permeable sinusoidal niches, facilitating HSPC mobilization.48 Consistently, the less permeable endosteal arterioles harbor quiescent HSCs with low reactive oxygen species (ROS) content, whereas HSCs in the proximity of sinusoidal vessels are more heterogenous, with sinusoidal ROSlow HSCs being aligned predominantly with megakaryocytes.44,48
Several megakaryocyte-derived factors contribute to regulating HSC quiescence, including platelet factor 4 (PF4; CXCL4) and transforming growth factor (TGF)-β, and megakaryocytes promote FGF1-dependent HSC expansion under stress conditions.49,50 PF4 enhances TM-dependent PC activation without changing the generation of antifibrinolytic TAFI.51,52 Nonmyelinating Schwann cells also provide a niche for hibernating BM HSCs via TGF-β production.53 These mechanisms involving mediators released from megakaryocytes and other cell types are an important aspect of how the hemostatic system contributes to HSC maintenance in BM niches associated with less permeable vessels.
Components of the anticoagulant pathway are detectable not only in the lumen of the BM vasculature, but also in the extravascular space of the BM niche. EPCR is expressed by HSCs,11 endothelial cells,8 and cells of the mesenchymal lineage.54 TM is expressed by BM HSPCs and by endothelial cells, as well as stromal, macrophage, and B-cell precursor populations.55 PC immunoreactivity is found associated not only with BM endothelial cells, but also surrounding EPCR+ HSCs.56 Although coagulation can be triggered directly by endosteal osteoclasts35 or megakaryocytes,57 it remains an open question whether PC is activated by extravascular components of the anticoagulant pathway or originates from the lumen of more permeable sinusoidal vessels to regulate HSCs in their niche.
Regulation of HSCs by the anticoagulant pathway
EPCR as a marker of dormant HSCs
Since the discovery that EPCR identifies HSCs in the murine BM,11 surface EPCR expression has been integrated into LSK-SLAM–sorting schemes to isolate HSCs for definition of their functional properties. Single-cell next-generation sequencing has recently documented considerable heterogeneity of HSCs and confirmed that high EPCR expression is a general property of quiescent HSCs with multilineage reconstitution potential.58 In murine BM, vitamin A signaling maintains a small (≈20%) subpopulation of HSCs with very low expression of c-Myc and high expression of Gpcr5c and EPCR.59 Stress-induced activation of these dormant HSCs is prevented by treatment with all-trans retinoic acid that antagonizes increased HSC transcription of c-Myc, protein translation, and generation of reactive oxygen species (ROS). This HSC exit from dormancy is paralleled by a gradual loss of EPCR expression and upregulation of biosynthetic pathways, as well as c-Myc and the megakaryocyte markers integrin α2b (CD41) and CXCL4 (PF4).
Consistently, comparing different HSC-sorting schemes by single-cell next-generation sequencing profiling has demonstrated that high levels of EPCR and absence of integrin α2b characterize HSCs with low proliferation and differentiation and the highest multilineage repopulation potential.58 More proliferative populations have reduced EPCR expression and upregulate the megakaryocyte markers integrin α2b and VWF.58 Index sorting for high expression of CD150 and EPCR also isolates cells with delayed proliferation in vitro.60 In addition, VWF is found in an integrin α2b− EPCR-expressing HSC with megakaryocyte- and myeloid cell–differentiation bias25 and long-term repopulation potential.61
EPCR is also a reliable surface marker for the prospective isolation of HSCs from different mouse strains and under stress conditions.62 EPCR expression can be used to identify and isolate HSCs from the fetal liver.61,63 Furthermore, human cord blood expanded with the HSC renewal-promoting pyrimido-indole derivative UM171 yields EPCR+ CD34+ HSCs with multilineage repopulation potential in immune-deficient mice.64 During development, HSCs appear in day-11 mouse embryos in the aorta–gonad–mesonephros region where HSC precursors branch off from the endothelial cell lineage. High expression of EPCR identifies the earliest VE-cadherin+ CD31+ CD45− HSC percursors expressing the embryonic HSC marker integrin α2a and exhibiting single-cell reconstitution potential.65 The close developmental relationship between endothelial and hematopoietic cells is further reflected by the recent demonstration that EPCR expression marks vessel wall–resident endothelial stem cells that give rise to endothelial cells and vascular pericytes in adult animals.66 This finding challenges the paradigm that pericytes are of mesenchymal origin in postnatal angiogenesis. In addition, EPCR+ pluripotent mammary gland stem cells can reconstitute all lineages of the mammary epithelium.10 Because EPCR function supports stem cell activity in poor-prognosis human breast cancer,9 it is also of interest for leukemia development how EPCR transmits cues from the BM niche environment to support HSC maintenance, chemotherapy resistance, proliferation, and differentiation.
EPCR as a functional regulator of HSCs
FVIII-deficient mice develop altered trabecular bone structures, suggesting that activation of coagulation participates in bone remodeling and osteoblast function in the BM cavity.67 A similar phenotype of PAR1-deficient mice and the finding that PAR1 is expressed in mesenchymal cells and osteoblasts indicate that extravascular coagulation regulates the BM niche, at least in part, through cell signaling. Consistently, proliferative BM CD34+ HSPCs and circulating HSPCs are increased in FVIII-knockout mice, and FVIII and PAR1 deficiencies similarly cause splenomegaly due to extramedullary hematopoiesis.67 Age-dependent BM failure leading to splenomegaly also develops in EPCR-R84A mice mutated to specifically impair PC binding and activation, causing a prothrombotic phenotype.68 The progressive BM failure in FVIII-deficient mice with reduced thrombin generation, as well as in hyperthrombotic EPCR-R84A mice with reduced EPCR ligand binding, suggests a common pathway that is dependent on mesenchymal stromal cells, including osteoblasts, whose proliferation is known to be dependent on EPCR signaling.69 It will be of interest to confirm this regulatory role of aPC signaling in osteoblasts or other stromal cells with PAR1 mutant mice devoid of aPC but not thrombin signaling.4
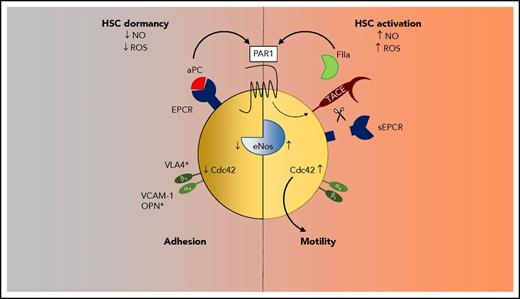
However, loss of PAR1 or EPCR function on hematopoietic cells also contributes to BM failure67,68 and lower chemotherapy resistance.56 Additional evidence indicates that HSC-expressed EPCR does not primarily regulate coagulation, but rather supports HSC BM retention through PAR1 signaling (Figure 2).56 Treatment with an HSC adhesion-blocking integrin α4 (VLA4) antibody or with an inhibitory antibody to EPCR rapidly mobilizes EPCR+ HSPC into the circulation, and EPCR-deficient mice have higher numbers of circulating HSPCs. Adhesion of HSCs requires the inactivation of the small GTPase Cdc42, which shows increased activity in aged HSCs.70 EPCR+ HSCs are characterized by a polar expression of Cdc42 and low levels of active GTP-loaded Cdc42.56 Conversely, loss of EPCR function by antibody blockade or deletion reduces Cdc42 polarity and increases Cdc42 activity. Stimulation of HSCs with aPC increases integrin α4 ligand affinity dependent on EPCR expression, supporting a pathway in which aPC-EPCR signaling limits Cdc42 activation and increases integrin α4–dependent adhesion of HSCs in BM niches. Additional studies are required to conclusively determine whether aPC-EPCR signaling primarily serves HSC BM niche retention or has additional functions in rendering HSCs quiescent.
Biased PAR1 signaling regulates HSC quiescence and mobility. PAR1 activation by EPCR-aPC promotes inhibition of endothelial NO synthase (eNOS), low NO and ROS levels, and suppression of Cdc42 activity and increased VLA (integrin α4)–mediated adhesion to niche ligands. Biased thrombin-PAR1 signaling releases EPCR by TACE shedding and upregulates NO, Cdc42 activity, and motility.
Biased PAR1 signaling regulates HSC quiescence and mobility. PAR1 activation by EPCR-aPC promotes inhibition of endothelial NO synthase (eNOS), low NO and ROS levels, and suppression of Cdc42 activity and increased VLA (integrin α4)–mediated adhesion to niche ligands. Biased thrombin-PAR1 signaling releases EPCR by TACE shedding and upregulates NO, Cdc42 activity, and motility.
Consistent with EPCR-PAR1 contributing to functional preservation of primitive HSCs, inhibition of EPCR or PAR1 deficiency sensitizes mice to myeloablative stress. Regulation of HSC function by EPCR-PAR1 signaling involves suppression of nitric oxide (NO) production, which increases HSC motility and reduces adhesion. EPCR deficiency prevents aPC-induced suppression of NO levels in HSPCs, and inhibitory endothelial NO synthase (Nos3) Thr495 phosphorylation is promoted by active aPC but not the alternative EPCR ligand FVIIa. Competitive BM-transplantation experiments demonstrate that in vivo pretreatment of WT BM with a nonspecific NO synthase inhibitor, even in mice deficient for EPCR, increases donor chimerism, implicating regulation of NO production in HSC engraftment. EPCR-aPC signaling also counteracts the activating Nos3 Ser1177 phosphorylation by thrombin, indicating that NO production is a target for biased PAR1 signaling in HSPCs. Consistent with a primary regulation of HSC retention, in vitro–differentiation assays show no HSPC lineage bias in EPCR-dysfunctional mice, whereas platelet numbers were low.68 Low circulating platelets counts have been documented for other hyperthrombotic mouse models and are likely caused by increased platelet turnover.71,72
Stress responses of HSCs and the BM niche
Coagulation and fibrinolysis in the response to BM stress
Acute and chronic bleeding is a major challenge requiring increased hematopoiesis to restore blood loss. Injury and infection cause coordinated activation of the complement and coagulation system in the context of cell damage and the release of inflammatory mediators implicated in the regulation of HSPCs, including sphingosine-1 phosphate, antimicrobial LL37, C3a, and C5a.73 The complement and coagulation cascades are connected at several levels and may cooperate in HSC maintenance and egress to the blood. The mannose-binding lectin pathway activates mannose-binding lectin–associated serine proteases (MASP-1 and MASP-2) that can directly activate prothrombin. Complement activation is required for granulocyte colony-stimulating factor (G-CSF) mobilization of HSPCs, and C3a, but not the TAFI-degradation product desArgC3a, stimulates HSC C3a receptors and motility.74 C3 conversion promotes platelet activation, whereas C5 activation stimulates TF activity on myeloid cells12 and also contributes to HSPC egress. Extracellular nucleotides released in the context of cell damage also regulate HSPCs75 and are known to stimulate the release of TF procoagulant extracellular vesicles from macrophages, coupling coagulation and inflammation.76
Coagulation activation in the periphery due to severe trauma or sepsis may also provide a signal that activates HSPCs in the BM niche. Experimental injection of high concentrations of thrombin shows that thrombin activity can enter the BM niche and rapidly mobilize HSCs.56 In this context, thrombin-PAR1 signaling increases CXCL12 levels in blood, releases CXCL12 from mesenchymal stromal cells, and, thereby, diminishes the CXCL12-CXCR4 chemokine signaling crucial for HSC BM retention.39 In addition, HSC PAR1 activation by thrombin promotes ADAM17 (TACE, CD156b)–dependent shedding of EPCR, upregulates HSC expression of CXCR4 and PAR1, and mobilizes PAR1-expressing HSPCs into the blood.56 As a consequence, mobilized HSPCs in the blood do not express EPCR, but following their transplantation and BM repopulation, they reexpress EPCR.56 HSC PAR1 activation by thrombin induces Nos3 Ser1177 phosphorylation and NO production, Cdc42 activation, diminished integrin α4 affinity, increased HSC mobility, and Nos3-dependent HSC BM egress. Thrombin-induced HSC activation may also be relevant in the context of inflammation that locally or systemically triggers TF-dependent coagulation.77
G-CSF is a potent cytokine that is used to clinically induce neutrophil maturation and mobilization of dormant HSCs into the circulation.78 G-CSF injection leads to a transient procoagulant state77,79,80 that may contribute to HSC mobilization, because, similar to thrombin injection,56 G-CSF also decreases BM CXCL12 levels and upregulates CXCR4 on HSCs.81 PAR1 is essential for CXCL12-mediated HSPC migration and chemotaxis,56 and G-CSF–mobilized human CD34+ HSPCs have elevated PAR1 levels compared with steady-state BM HSPCs.82 In addition to coagulation, activation of the fibrinolytic system is crucial for G-CSF–induced mobilization of HSPCs. Plasmin-dependent matrix metalloproteinase 9 (MMP9) activation causes CXCL12 shedding from mesenchymal cells, increased levels of chemotactic CXCL12 in blood, upregulation of HSPC CXCR4 in the BM, and mobilization of HSPCs into the blood.83 Another component of the fibrinolytic system, the urokinase receptor (uPAR), is expressed by myeloid progenitor populations that are mobilized by G-CSF.84 Release of uPAR by phospholipase D–mediated cleavage of the glycosylphosphatidylinositol anchor in response to G-CSF increases circulating levels of soluble uPAR, which is known to interact with integrins, including integrin α4, to antagonize HSPC CXCR4 signaling and to promote HSPC motility and mobilization.84 In addition, the noncanonical roles of fibrinolysis contribute to HSPC functions in postinfarction repair of the myocardium.85 Thus, cell adhesion and the CXCL12-CXCR4 HSC signaling axis are targets for regulation by coagulation and fibrinolytic systems.
In addition to myeloablative protection by anticoagulant EPCR–aPC–PAR1 signaling,56 fibrinolysis assures recovery from chemotherapy-induced stress. Plasmin generation by tissue plasminogen activator has fibrin-independent functions in MMP-mediated degradation of BM matrix and VCAM (CD106),86 and activation of MMP9 mediates release of KitL and, thereby, enables HSC proliferation and differentiation in BM regeneration.87 This MMP9-dependent HSC activation facilitates motility and reconstitution of stem and progenitor cell pools in vascular niches of the BM.88 The receptor for KitL, cKit (CD117), can also be proteolytically removed by MMP and other proteases that, thereby, act synergistically during mobilization.89
Hematopoietic progenies in the restoration of HSC niches
BM niches receive a variety of signals during infections and inflammation that act on HSPCs and include G-CSF initiating “emergency” granulopoiesis, inflammatory mediators, tumor necrosis factor, interleukin-1, and types I and II interferons.90,91 Progeny of hematopoietic cells with procoagulant potential are also directly involved in the regulation of HSPC fate and in restoring HSC quiescence after insults. BM macrophages responding to G-CSF play a pivotal role in maintaining osteoblast function in the endosteum,92,93 and CD169+ BM macrophages regulate mesenchymal cells in vascular niches.94 Because inflammatory mediators upregulate TF in myelo-monocytic cells, extravascular coagulation and its signaling functions may have incompletely characterized roles in macrophage-dependent HSPC BM niche regulation during infection.
In addition, megakaryocytes play a central role in restoring BM function after myeloablative therapy and during infection. Stimulation of mice with the TLR3 ligand polyinosinic-polycytidylic acid, mimicking viral infections, triggers a type I interferon response in HSPCs that rapidly matures megakaryocyte-committed progenitors independently of differentiation through erythrocyte–megakaryocyte progenitor stages.95 Although the bulk of megakaryopoiesis under steady-state is sustained by differentiation from progenitors in BM and lung,96 “emergency” megakaryopoiesis activates an integrin α2b–expressing progenitor residing in the phenotypic BM HSC compartment.95 Proteome analysis of these activated progenitors also indicates that components of coagulation pathways are expressed during this rapid replenishing of megakaryocyte pools, assuring platelet production for increased demand in the periphery.95,97 Lineage tracing provided evidence that, during steady-state hematopoiesis, megakaryocytes also differentiate directly from lineage-committed progenitors residing in the HSC compartment.97
BM megakaryocytes are also pivotal for restoring HSC quiescence after BM insults. Megakaryocyte depletion results in a loss of HSC quiescence.49,50 Megakaryocytes suppress HSC proliferation by secreting CXCL4 (PF4) and increasing active TGF-β in the sinusoidal niche. In chemotherapy-induced BM stress, megakaryocyte-derived FGF1 also expands HSCs and, thus, restores BM function. In addition, megakaryocytes are crucial for limiting BM radiation injury by secreted PDGF-BB acting on osteoblasts, restoring the endosteal niche.98 Mature polyploid megakaryocytes also express FV, FX, and prothrombin, can generate thrombin, and, thereby, activate the crucial matrix niche factor (ie, osteoblast-derived osteopontin).57 These data indicate that the extravascular coagulation activation by megakaryocytes has overlapping functions with bone-remodeling osteoclasts. Thus, coagulation and fibrinolysis contribute to HSC mobilization, as well as to restoration of HSC maintenance in the BM.
Therapeutic implications
Proof-of-principle experiments show that therapeutic targeting of the anticoagulant pathway has beneficial effects in reducing BM stress and the lethal effects associated with radiation injury.55 Retroviral insertion mutagenesis–induced upregulation of TM in mice HSPCs conferred a marked protection from lethal irradiation. Conversely, HSPCs expressing a dysfunctional TM (in TMPro mice) were more susceptible to radiation injury. In addition to these incompletely understood functions of TM on HSPCs, stromal cell TM-dependent activation of the anticoagulant pathway was crucial for survival from lethal irradiation. TM-dysfunctional mice were rescued by a PC variant that did not require activation by TM. Mice were also less susceptible to whole body irradiation when the balance was tipped toward anticoagulation by treatment with a recombinant preparation of soluble TM or with mutants of aPC retaining anticoagulant, but not the canonical aPC-PAR1, signaling function. However, antithrombotic strategies preventing contact pathway–dependent FXI activation or standard heparin therapy provided little benefit.
Despite this demonstrated therapeutic efficacy of anticoagulant strategies in restoring BM function following radiation injury, it is unclear whether antithrombotic therapy targeting platelets and coagulation has an impact on HSC niches under steady-state or other stress conditions. Perivascular location of megakaryocytes in the BM is regulated by glycoprotein 1bα, rather than platelet receptors targeted by antithrombotic therapy,99 indicating that antiplatelet therapy may not interfere with critical niche functions of megakaryocytes. Platelets and possibly megakaryocytes in the BM niches play important roles in coagulation, by supporting the FVa-FXa prothrombinase complex and contact phase–independent FXI amplification in vascular inflammation.100 Although thrombocytopenia caused by impaired megakaryopoiesis likely influences HSC maintenance, a better understanding of the roles of the contact pathway and FXI in BM niche regulation should be of interest for the development of safer anticoagulant strategies targeting these pathways.
The effects of traditional and new anticoagulants on intravascular coagulation are well characterized, but their functions in extravascular locations remain to be determined. Heparin therapy is unlikely to influence extravascular coagulation in the BM niche under steady-state, because the requisite target for heparin, antithrombin, is largely restricted to the blood and vascular interface. It will be of interest to better understand how new small molecule direct inhibitors diffuse into BM niches and how conventional oral vitamin K antagonists influence the synthesis and posttranslation modification of coagulation proteases synthesized by stromal cells in the BM microenvironment. Furthermore, the development and clinical availability of specific antagonists and modulators of PAR signaling may provide additional opportunities to favorably influence preservation and recovery of BM function beyond established antithrombotic therapies.
Acknowledgments
The authors thank their colleagues who participated in the original studies discussed in this review, which were supported by the National Institutes of Health, National Heart, Lung, and Blood Institute, the German Federal Ministry of Education and Research (BMBF 01EO1503), the Alexander von Humboldt Foundation (Germany), and the Israel Science Foundation.
Authorship
Contribution: T.S.N., T.L., and W.R. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Ruf, Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Langenbeckstr. 1, 55131 Mainz, Germany; e-mail: ruf@uni-mainz.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal