Key Points
Pathogen-inactivated platelets were noninferior in preventing bleeding only in intention-to-treat analysis.
In contrast to animal models, alloimmunization could not be prevented when using pathogen-inactivated platelets.
Abstract
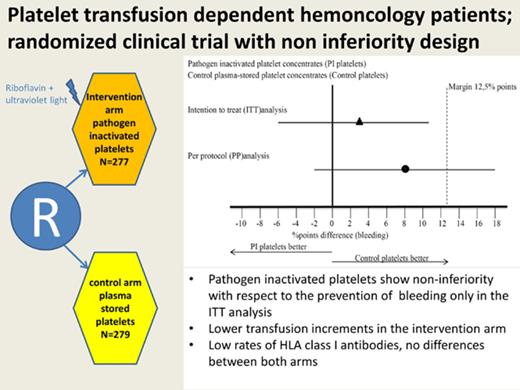
Pathogen inactivation of platelet concentrates reduces the risk for blood-borne infections. However, its effect on platelet function and hemostatic efficacy of transfusion is unclear. We conducted a randomized noninferiority trial comparing the efficacy of pathogen-inactivated platelets using riboflavin and UV B illumination technology (intervention) compared with standard plasma-stored platelets (control) for the prevention of bleeding in patients with hematologic malignancies and thrombocytopenia. The primary outcome parameter was the proportion of transfusion-treatment periods in which the patient had grade 2 or higher bleeding, as defined by World Health Organization criteria. Between November 2010 and April 2016, 469 unique patients were randomized to 567 transfusion-treatment periods (283 in the control arm, 284 in the intervention arm). There was a 3% absolute difference in grade 2 or higher bleeding in the intention-to-treat analysis: 51% of the transfusion-treatment periods in the control arm and 54% in the intervention arm (95% confidence interval [CI], −6 to 11; P = .012 for noninferiority). However, in the per-protocol analysis, the difference in grade 2 or higher bleeding was 8%: 44% in the control arm and 52% in the intervention arm (95% CI −2 to 18; P = .19 for noninferiority). Transfusion increment parameters were ∼50% lower in the intervention arm. There was no difference in the proportion of patients developing HLA class I alloantibodies. In conclusion, the noninferiority criterion for pathogen-inactivated platelets was met in the intention-to-treat analysis. This finding was not demonstrated in the per-protocol analysis. This trial was registered at The Netherlands National Trial Registry as #NTR2106 and at www.clinicaltrials.gov as #NCT02783313.
Introduction
There remains interest in the development of pathogen-inactivation techniques to complement the “multi-layered prevention strategy” to avert transfusion of blood products contaminated with currently known, as well as unknown, pathogens. The available pathogen-inactivation systems for platelet concentrates inactivate a broad array of viruses, bacteria, and parasites.1-4 Moreover, these techniques have also shown sufficient white cell inactivation to prevent transfusion-associated graft-versus-host disease and may also reduce the formation of HLA antibodies.5-7 If hemostatic efficacy of pathogen-inactivated platelets is sufficiently maintained, these advantages could favor the consideration to implement pathogen-inactivation technology. A meta-analysis of 12 randomized controlled trials concluded that transfusions with pathogen-inactivated platelet concentrates resulted in reduced transfusion increment, without hemostatic consequences or differences in patient survival.8 In 3 of these trials, riboflavin, also known as vitamin B2, with UV illumination (Mirasol pathogen-inactivation technology; Terumo BCT, Lakewood, CO) was used to inactivate pathogens.9-11 Despite the available data, it is insufficiently known whether Mirasol treatment in platelet concentrates results in an equivalent hemostatic effect in this vulnerable population. Because bleeding is considered the pivotal outcome for platelet-transfusion trials, we conducted a noninferiority randomized controlled trial comparing pathogen-inactivated platelet concentrates using the Mirasol technology with conventional untreated platelet concentrates, with percentage of transfusion-treatment periods in which the patient has World Health Organization (WHO) grade ≥2 bleeding as primary outcome.12 As a secondary outcome, we measured HLA antibody formation to determine whether pathogen-inactivated platelets are able to reduce alloimmunization in hemato-oncology patients.
Methods
The PREPAReS study (Pathogen Reduction Evaluation and Predictive Analytical Rating Score) was designed as a randomized multicenter noninferiority study using a parallel arm design with 1:1 randomization. The protocol was written by a steering committee and approved centrally and by site institutional review boards. The study met the criteria outlined in the Declaration of Helsinki (6th revision, 2008) and Good Clinical Practice guidelines. All patients gave written informed consent before the randomization procedure or any other study-related procedure. A detailed review of the protocol and methods used was published separately13 and is only briefly summarized here. The study was conducted in 3 countries, in 10 centers with hemato-oncology departments: 4 sites in The Netherlands, 5 in Canada, and 1 in Norway.
Eligibility criteria
Hemato-oncology patients with chemotherapy-induced thrombocytopenia aged ≥18 years were eligible for inclusion in the study if they were expected to require ≥2 platelet transfusions during a transfusion-treatment period (supplemental Figure 1, available on the Blood Web site). Patients presenting with a grade ≥2 bleeding before enrolment could only be enrolled with existing (ie, not new) bruises, whereas patients with grade ≥2 bleeding in organ systems other than skin could be enrolled only 14 days after resolution of the bleed. Other exclusion criteria included known immunological refractoriness to platelet transfusions, indications to use hyperconcentrated platelets, idiopathic thrombocytopenic purpura, pregnancy, microangiopathic thrombocytopenia, and known allergy to riboflavin or its photoactive products.
Stratification and randomization
Eligible patients were randomized to receive untreated plasma-stored platelet concentrates or pathogen-inactivated platelet concentrates using a centralized Web-based allocation tool. The random allocation schedule was prepared by a biostatistician not directly involved in the study using a 1:1 ratio and randomly varying block sizes of 2 to 6. Three stratification factors were applied: center, diagnosis (acute myeloid leukemia [AML] vs non-AML), and treatment (transplant vs no transplant). Patients could be randomized more than once if they had subsequent hospital admissions, and the statistical analysis accounted for multiple randomizations per individual.
Platelet products and transfusion policy
Platelet concentrates were all prepared from pooled buffy coats, resuspended in plasma, and leukoreduced by filtration.14 For pathogen inactivation, 35 mL (500 µM) of riboflavin was added to the pooled leukoreduced product and exposed to UV light (wavelength 280-315 nm) for 5-10 minutes, depending on the volume of the concentrate (total dose 6.2 J/mL), according to the manufacturer’s instructions. Platelet products were stored with gentle agitation at 20-24°C for up to 5 d in Canada and for a maximum of 7 days in The Netherlands and Norway.14 The products were composed of 5 buffy coats in The Netherlands, 4 buffy coats in Norway, and 4 or 5 buffy coats in Canada. The actual platelet content in the bags largely overlapped among the countries.14 An automated culture system was used to detect bacterial contamination, and products were issued as “negative to date.” Platelet concentrates were gamma irradiated as per the local protocol. In both treatment arms, patients received platelet transfusions prophylactically (platelet count–related prophylaxis [trigger 10 × 109/L] or intervention-related prophylaxis [trigger 50 × 109/L]) or as treatment for bleeding, using national and hospital guidelines. Red cell concentrates and plasma were transfused based on local protocols for transfusion thresholds, as well as at the treating physicians’ discretion.
Outcomes and clinical assessments
The primary study outcome was the proportion of transfusion-treatment periods in which the patient had a WHO grade ≥2 bleeding complication. The transfusion-treatment period started at the time of the first platelet transfusion after randomization and ended maximally 6 weeks after the first platelet transfusion or for 1 of the following reasons: patient was no longer thrombocytopenic (>7 days without requiring a platelet transfusion), hospital discharge, death, or request by the patient to discontinue; these data are shown in supplemental Figure 1. Secondary outcomes were 1- and 24-hour corrected count increments (CCIs), the frequency of transfusion failures (defined as 1-hour CCI <7.5 and 24-hour CCIs <4.5), the percentage of days within a transfusion-treatment period with bleeding grade ≥2, incidence of adverse transfusion reactions, transfusion requirement of red cells and platelets, platelet transfusion interval, and the proportion of patients with HLA alloimmunization. Data collection was performed by trained research staff at each site, and data were entered into the ProMISe (Project Manager Internet Server) database from 2 central research locations in Canada and The Netherlands. Bleeding symptoms, as well as all other clinical- and transfusion-related data, were monitored daily on all study patients, starting at randomization, up to a maximum of 6 weeks after the first platelet transfusion or the end of thrombocytopenia, as defined above. The study was not blinded, and bleeding assessments were performed by trained nonblinded research personnel. Hence, an adjudication process was used to assign each patient’s bleeding status to minimize bias. Bleeding adjudication, using the WHO bleeding scale, was done by 3 independent adjudicators blinded to the treatment allocation, in addition to the use of an automated algorithm.15 For HLA antibody detection, samples were collected weekly during hospitalization up until day 28, as well as a “late” sample that was obtained at approximately day 56, and tested in the Luminex assay (Luminex, Austin, TX) for the presence of single-antigen HLA antibodies at the Blood Systems Research Institute (San Francisco, CA).16
Statistical analyses
A pilot study showed that, on average, 50% of patients have grade ≥2 bleeding during their thrombocytopenic phase, confirming findings of earlier large platelet-transfusion studies.17-19 The study was designed as a noninferiority trial to test the null hypothesis that pathogen-inactivated platelet concentrates are worse than control platelets. The alternative hypothesis to be proven is that the pathogen-inactivated platelets perform similarly to control platelets within a prespecified margin with regard to the primary end point. Based on discussions with clinicians, as well as another large study using bleeding as an end point, we decided that a 12.5 percentage point increase as the upper limit of the 95% CI of the absolute difference in grade ≥2 bleeding between the treatment arms was an acceptable margin, acknowledging improved safety with regard to the transmission of pathogens.17 To assess the noninferiority hypothesis with a power of 80%, as well as adjustment (α and β spending) for predefined interim analyses, required a sample size of 578 (289 per arm). For safety reasons, frequent interim analyses were performed after every 60-patient transfusion-treatment period randomized using a flexible stopping rule based on α and β spending functions, allowing stopping for noninferiority or futility.20 Before unblinding and starting the final analyses, a statistical analysis plan was written and agreed upon by the steering committee. The analysis of the primary end point, as well as the majority of secondary end points, was performed using 3 approaches: intention-to-treat (ITT), the per-protocol population, and the per-protocol–only population (supplemental Table 1). The primary effect parameter was estimated according to a generalized estimating equation approach using a generalized linear model with identity link and independence working correlation. The dependent variable was the yes/no indicator of having at least 1 grade ≥2 bleed during a transfusion-treatment period. Covariates in the model were the treatment arm, the treatment period number (dichotomized as first or later), and the interaction between these 2 covariates. The 1-hour and 24-hour CCIs were analyzed using a linear mixed model using a random intercept per patient and a random intercept per treatment period to take into account the correlations between transfusions within treatment periods as well as between treatment periods within patients. Covariates were treatment arm, the number of the transfusion within the treatment period, the interaction between both and the pretransfusion count. The platelet transfusion interval was analyzed with a mixed Poisson model with the number of transfusions per treatment period as dependent variable, the treatment arm as covariate, the log of the duration of the treatment period as an offset parameter and a random intercept per patient. The other numerical secondary outcomes that were measured only once per treatment period were compared based on the mean value per group with a similar generalized estimating equation approach as for the primary outcome only now using a general linear model. For the analysis of the alloimmunization data, for patients with multiple randomizations, only results of the first randomizations were used. Patients were considered to be alloimmunized if ≥1 sample taken during the 56 days after randomization had a signal >5 standard deviations (SDs) above the normalized background signal. We calculated Kaplan-Meier curves for time to alloimmunization and compared both groups using a risk ratio for cumulative event probabilities estimated at 60 days. All statistical analyses were performed using IBM SPSS Statistics (version 23).
Study oversight
Safety aspects of the study were closely watched by a Data Safety Monitoring Board (DSMB). Interim analyses after every 60 randomized patients were evaluated by the DSMB. The study was monitored for quality and regulatory compliance. The monitoring frequency depended on inclusion rates and findings from earlier visits. The authors vouch for the integrity of the data and analyses reported. The study was sponsored by Sanquin Blood Supply and registered at the Netherlands National Trial Registry under number NTR2106, as well as at clinicaltrials.gov under number NCT02783313.
Results
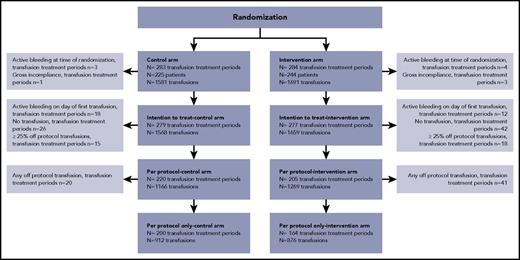
From November 2010 until April 2016, randomization of 567 transfusion-treatment periods took place in 469 patients. In November 2015, after analyzing 433 treatment periods, the DSMB advised to stop recruiting patients, because analysis of the ITT population permitted a conclusion of noninferiority for the primary end point. In close collaboration with the ethics review board, because there were no safety issues involved, the steering committee decided to continue patient accrual to reach the originally planned power of the study for the secondary end points, especially alloimmunization. Of the randomized transfusion-treatment periods, 11 were excluded from further analyses, because the patient had an active grade ≥2 bleeding (n = 8) at randomization or there was a gross lack of study compliance (n = 3; Figure 1). Thus, the ITT analyses were performed on 556 transfusion-treatment periods. For the per-protocol analyses, the data set consisted of 425 treatment periods after excluding patients who actively bled on the day of the first transfusion, did not receive any transfusion, or received ≥25% off-protocol transfusions (Figure 1). Randomization successfully balanced the most important risk factors for bleeding (Table 1).
Flow diagram of the study. In total, 567 randomizations occurred in 469 patients. The ITT analysis set consisted of all transfusion-treatment periods in which the patient met the inclusion and exclusion criteria. In the event of >25% off-protocol transfusions or no transfusions, these episodes were analyzed “as randomized.” For the ITT analysis, the first day of observation was the day of randomization. The per-protocol set consisted of all “on-protocol” episodes (ie, episodes in which the percentage of off-protocol transfusions exceeded 25% before the first grade ≥2 bleeding event or episodes without transfusions were excluded). For the per-protocol analysis, the first day of observation was the day of the first platelet transfusion. The per-protocol–only analysis set consisted of all transfusion-treatment periods in which only on-protocol transfusions are administered before a grade ≥2 bleeding occurred; the first day of observation was the day of the first platelet transfusion.
Flow diagram of the study. In total, 567 randomizations occurred in 469 patients. The ITT analysis set consisted of all transfusion-treatment periods in which the patient met the inclusion and exclusion criteria. In the event of >25% off-protocol transfusions or no transfusions, these episodes were analyzed “as randomized.” For the ITT analysis, the first day of observation was the day of randomization. The per-protocol set consisted of all “on-protocol” episodes (ie, episodes in which the percentage of off-protocol transfusions exceeded 25% before the first grade ≥2 bleeding event or episodes without transfusions were excluded). For the per-protocol analysis, the first day of observation was the day of the first platelet transfusion. The per-protocol–only analysis set consisted of all transfusion-treatment periods in which only on-protocol transfusions are administered before a grade ≥2 bleeding occurred; the first day of observation was the day of the first platelet transfusion.
Patient characteristics
| . | Control (n = 279)* . | Intervention (n = 277)* . |
|---|---|---|
| Male/female, n | 191/88 | 188/89 |
| Age, mean ± SD, y | 54 ± 12 | 54 ± 12 |
| Body surface area, mean ± SD, m2 | 1.97 ± 0.25 | 2.00 ± 0.24 |
| Enlarged spleen, n (%) | 17 (6.1)† | 31 (11) |
| Multiple inclusions, n (%) | 57 (20) | 39 (14) |
| Diagnosis, n (%) | ||
| AML | 132 (47.3) | 133 (48.0) |
| Acute lymphoblastic leukemia | 25 (9.0) | 24 (8.7) |
| Mantle cell lymphoma | 13 (4.7) | 14 (5.1) |
| Non-Hodgkin lymphoma | 41 (15.0) | 38 (13.7) |
| Multiple myeloma | 43 (15.0) | 45 (16.2) |
| Chronic leukemia | 3 (1.1) | 0 (0) |
| Other | 22 (7.9) | 23 (8.3) |
| Treatment, n (%) | ||
| Remission induction chemotherapy | 119 (42.7) | 116 (41.9) |
| Consolidation chemotherapy | 32 (11.5) | 35 (12.6) |
| Autologous stem cell transplantation | 101 (36.2) | 103 (37.2) |
| Allogeneic stem cell transplantation | 25 (8.9) | 16 (5.8) |
| Other | 2 (0.7) | 7 (2.5) |
| Laboratory values at randomization, mean ± SD | ||
| Platelet count, 109/L | 87 ± 100 | 79 ± 75 |
| Hemoglobin, g/L | 81 ± 29 | 82 ± 24 |
| Activated partial thromboplastin time, s | 29 ± 7.9 | 29 ± 8.7 |
| Prothrombin time, s | 12 ± 2.2 | 12 ± 1.9 |
| Fibrinogen, g/L | 3.7 ± 1.3 | 3.6 ± 1.4 |
| Medication and medical history, n (%) | ||
| Anticoagulant/antiplatelet therapy | 30 (10.8) | 31 (11) |
| Bleeding | 67 (24) | 72 (26) |
| Infection | 26 (9.3) | 27 (10) |
| Prior platelet transfusion | 181 (67) | 162 (60) |
| Prior red cell transfusion | 197 (71) | 191 (69) |
| Prior stem cell transplant procedure | 22 (7.9)† | 9 (3.2) |
| Prior pregnancy | 61 (22) | 66 (24) |
| . | Control (n = 279)* . | Intervention (n = 277)* . |
|---|---|---|
| Male/female, n | 191/88 | 188/89 |
| Age, mean ± SD, y | 54 ± 12 | 54 ± 12 |
| Body surface area, mean ± SD, m2 | 1.97 ± 0.25 | 2.00 ± 0.24 |
| Enlarged spleen, n (%) | 17 (6.1)† | 31 (11) |
| Multiple inclusions, n (%) | 57 (20) | 39 (14) |
| Diagnosis, n (%) | ||
| AML | 132 (47.3) | 133 (48.0) |
| Acute lymphoblastic leukemia | 25 (9.0) | 24 (8.7) |
| Mantle cell lymphoma | 13 (4.7) | 14 (5.1) |
| Non-Hodgkin lymphoma | 41 (15.0) | 38 (13.7) |
| Multiple myeloma | 43 (15.0) | 45 (16.2) |
| Chronic leukemia | 3 (1.1) | 0 (0) |
| Other | 22 (7.9) | 23 (8.3) |
| Treatment, n (%) | ||
| Remission induction chemotherapy | 119 (42.7) | 116 (41.9) |
| Consolidation chemotherapy | 32 (11.5) | 35 (12.6) |
| Autologous stem cell transplantation | 101 (36.2) | 103 (37.2) |
| Allogeneic stem cell transplantation | 25 (8.9) | 16 (5.8) |
| Other | 2 (0.7) | 7 (2.5) |
| Laboratory values at randomization, mean ± SD | ||
| Platelet count, 109/L | 87 ± 100 | 79 ± 75 |
| Hemoglobin, g/L | 81 ± 29 | 82 ± 24 |
| Activated partial thromboplastin time, s | 29 ± 7.9 | 29 ± 8.7 |
| Prothrombin time, s | 12 ± 2.2 | 12 ± 1.9 |
| Fibrinogen, g/L | 3.7 ± 1.3 | 3.6 ± 1.4 |
| Medication and medical history, n (%) | ||
| Anticoagulant/antiplatelet therapy | 30 (10.8) | 31 (11) |
| Bleeding | 67 (24) | 72 (26) |
| Infection | 26 (9.3) | 27 (10) |
| Prior platelet transfusion | 181 (67) | 162 (60) |
| Prior red cell transfusion | 197 (71) | 191 (69) |
| Prior stem cell transplant procedure | 22 (7.9)† | 9 (3.2) |
| Prior pregnancy | 61 (22) | 66 (24) |
n = number of transfusion-treatment periods.
P < .05.
Bleeding
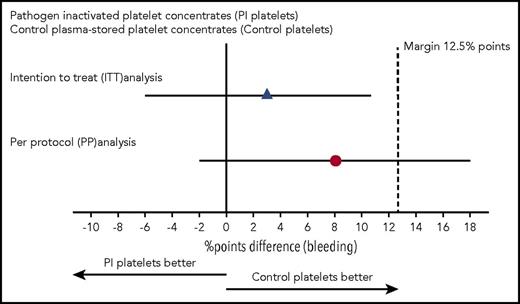
In the ITT analysis, in 51% of the transfusion-treatment periods in the control arm, the patient experienced a WHO grade ≥2 bleeding vs 54% in the intervention arm. The upper boundary of the 95% CI of the difference between these 2 percentages did not exceed 12.5 percentage points, hence meeting the noninferiority criterion (Table 2). However, for the per-protocol analysis, 44% of patients receiving standard platelet products had a grade ≥2 bleeding vs 52% in the intervention arm (Table 2). The upper limit of the 95% CI of this difference exceeded the prespecified limit, so the noninferiority criterion was not met (Figure 2). When looking at the percentage of bleeding days, there was no significant difference between the arms, irrespective of the analysis used. Also, when considering the highest bleeding grade, we saw no differences between the control and intervention arms. A further subanalysis was performed for patients receiving only on-protocol transfusions, which showed similar outcome as the per-protocol analysis (supplemental Table 2).
Bleeding complications
| . | Control . | Intervention . |
|---|---|---|
| ITT analysis | ||
| Transfusion-treatment periods, n | 279 | 277 |
| Primary end point | ||
| WHO grade 2, 3 or 4 bleeding, n (%)* | 143 (51) | 150 (54) |
| Days from randomization to first grade 2, 3, or 4 bleeding, median (IQR), n | 5 (2-8) | 5.5 (2-9) |
| Days with grade 2, 3, or 4 bleeding, median (IQR), %† | 3 (0-14) | 5 (0-15) |
| Days with grade 2, 3, or 4 bleeding, median (IQR), n | 1 (0-2) | 1 (0-2) |
| Highest grade of bleeding, n (%) | ||
| None or grade 1 | 136 (49) | 127 (46) |
| Grade 2 | 131 (47) | 139 (50) |
| Grade 3 | 6 (2) | 5 (2) |
| Grade 4 | 6 (2) | 6 (2) |
| Per-protocol analysis | ||
| Transfusion-treatment periods, n | 220 | 205 |
| Primary end point | ||
| WHO grade 2, 3, or 4 bleeding, n (%)‡ | 97 (44) | 107 (52) |
| Days from first transfusion to first grade 2, 3, or 4 bleeding, median (IQR), n | 3 (1-5) | 3 (1-5) |
| Days with grade 2, 3, or 4 bleeding, median (IQR), § | 0 (0-15) | 4 (0-17) |
| Days with grade 2, 3, or 4 bleeding, median (IQR) n | 0 (0-2) | 1 (0-2) |
| Highest grade of bleeding, n (%) | ||
| None or grade 1 | 123 (56) | 98 (48) |
| Grade 2 | 87 (40) | 102 (50) |
| Grade 3 | 4 (2) | 2 (1) |
| Grade 4 | 6 (3) | 3 (2) |
| . | Control . | Intervention . |
|---|---|---|
| ITT analysis | ||
| Transfusion-treatment periods, n | 279 | 277 |
| Primary end point | ||
| WHO grade 2, 3 or 4 bleeding, n (%)* | 143 (51) | 150 (54) |
| Days from randomization to first grade 2, 3, or 4 bleeding, median (IQR), n | 5 (2-8) | 5.5 (2-9) |
| Days with grade 2, 3, or 4 bleeding, median (IQR), %† | 3 (0-14) | 5 (0-15) |
| Days with grade 2, 3, or 4 bleeding, median (IQR), n | 1 (0-2) | 1 (0-2) |
| Highest grade of bleeding, n (%) | ||
| None or grade 1 | 136 (49) | 127 (46) |
| Grade 2 | 131 (47) | 139 (50) |
| Grade 3 | 6 (2) | 5 (2) |
| Grade 4 | 6 (2) | 6 (2) |
| Per-protocol analysis | ||
| Transfusion-treatment periods, n | 220 | 205 |
| Primary end point | ||
| WHO grade 2, 3, or 4 bleeding, n (%)‡ | 97 (44) | 107 (52) |
| Days from first transfusion to first grade 2, 3, or 4 bleeding, median (IQR), n | 3 (1-5) | 3 (1-5) |
| Days with grade 2, 3, or 4 bleeding, median (IQR), § | 0 (0-15) | 4 (0-17) |
| Days with grade 2, 3, or 4 bleeding, median (IQR) n | 0 (0-2) | 1 (0-2) |
| Highest grade of bleeding, n (%) | ||
| None or grade 1 | 123 (56) | 98 (48) |
| Grade 2 | 87 (40) | 102 (50) |
| Grade 3 | 4 (2) | 2 (1) |
| Grade 4 | 6 (3) | 3 (2) |
IQR, interquartile range.
Difference is 3 percentage points, 95% CI (−6 to 11), P = .012 for noninferiority. After correcting for stratification factors (center, diagnosis AML/non-AML, and treatment phase conventional/stem cell), the difference is 1 percentage point, 95% CI (−6 to 9), P = .002 for noninferiority.
P = .535 for superiority of mean percentages.
Difference is 8 percentage points, 95% CI (−2 to 18), P = .19 for noninferiority. After correcting for stratification factors (center, diagnosis AML/non-AML, and treatment phase conventional/stem cell), the difference is 10 percentage points, 95% CI (1-19), P = .29 for noninferiority.
P = .538 for superiority of mean percentages.
Noninferiority plot comparing the difference in percentage of transfusion-treatment periods with WHO grades 2, 3, and 4 bleeding in the intervention and control arms. The point estimates of the difference in percentage points and their 95% CIs are displayed for the ITT analysis and the per-protocol analysis. The dashed vertical line shows the predefined margin of 12.5 percentage points. For the ITT analysis, the noninferiority criterion is met. For the per-protocol analysis, the 95% CI exceeds the margin of 12.5% points, and the noninferiority criterion is not satisfied.
Noninferiority plot comparing the difference in percentage of transfusion-treatment periods with WHO grades 2, 3, and 4 bleeding in the intervention and control arms. The point estimates of the difference in percentage points and their 95% CIs are displayed for the ITT analysis and the per-protocol analysis. The dashed vertical line shows the predefined margin of 12.5 percentage points. For the ITT analysis, the noninferiority criterion is met. For the per-protocol analysis, the 95% CI exceeds the margin of 12.5% points, and the noninferiority criterion is not satisfied.
Transfusions
Most platelet transfusions were given prophylactically (supplemental Tables 3 and 4). The pretransfusion platelet count was ∼15 × 109/L, with no differences between the 2 arms. The platelet content in the products was about equal13,14 (supplemental Tables 3 and 4). Storage time was comparable, with 16%-19% of the concentrates being stored for 6 or 7 days. The percentage of off-protocol transfusions in the intervention arm was 19.5% vs 11.6% in the control arm (P = .02). Off-protocol transfusions were denoted as “other” (eg, hyperconcentrated platelet products, platelets in additive solution in the control arm, and untreated platelets in the intervention arm). All transfusion-increment parameters were significantly lower for pathogen-inactivated platelet concentrates vs untreated platelets. In the intervention arm, the count increments and CCIs were ∼50% lower than the values in the control platelets arm, resulting in frequent transfusion “failures” (Table 3), a higher number of platelet transfusions, and a shorter platelet transfusion interval (Table 4). There were no differences in the number of red cell units and plasma units transfused in either arm for the ITT and per-protocol analyses (Table 4).
Platelet transfusion increment
| . | Control . | Intervention . | P . |
|---|---|---|---|
| ITT analysis | |||
| Platelet transfusions, n | 1568 | 1659 | |
| Efficacy parameters, mean ± SD | |||
| Count increment 1 h, 109/L | 25 ± 14 (n = 848) | 13 ± 8 (n = 997) | |
| CCI 1 h | 13 ± 7 (n = 848) | 8 ± 5 (n =9 97) | <.001 |
| Count increment 24 h, 109/L | 14 ± 14 (n = 953) | 8 ± 9 (n = 1007) | |
| CCI 24 h | 7 ± 7 (n = 953) | 4 ± 4 (n = 1007) | <.001 |
| Transfusion failure rate, median (IQR) | |||
| CCI 1 h, <7.5 | 0 (0-0.08) | 0.50 (0.09-0.75) | <.001 |
| CCI 24 h, <4.5 | 0 (0-0.33) | 0.50 (0.20-0.83) | <.001 |
| CCI 24 h, ≤0 | 0 (0-0) | 0 (0-0.08) | .013 |
| Per-protocol analysis | |||
| Platelet transfusions, n | 1395 | 1391 | |
| Efficacy parameters, mean ± SD | |||
| Count increment 1 h, 109/L | 25 ± 14 (n = 796) | 12 ± 8 (n = 868) | |
| CCI 1 h | 13 ± 7 (n = 796) | 7 ± 4 (n = 868) | <.001 |
| Count increment 24 h, 109/L | 14 ± 14 (n = 895) | 7 ± 8 (n = 897) | |
| CCI 24 h | 8 ± 7 (n = 895) | 4 ± 4 (n = 897) | <.001 |
| Transfusion failure rate, median (IQR) | |||
| CCI 1 h, <7.5 | 0 (0-0.02) | 0.50 (0.16-0.89) | <.001 |
| CCI 24 h, <4.5 | 0 (0-0.33) | 0.50 (0.18-0.93) | <.001 |
| CCI 24 h, ≤0 | 0 (0-0) | 0 (0-0.02) | .014 |
| . | Control . | Intervention . | P . |
|---|---|---|---|
| ITT analysis | |||
| Platelet transfusions, n | 1568 | 1659 | |
| Efficacy parameters, mean ± SD | |||
| Count increment 1 h, 109/L | 25 ± 14 (n = 848) | 13 ± 8 (n = 997) | |
| CCI 1 h | 13 ± 7 (n = 848) | 8 ± 5 (n =9 97) | <.001 |
| Count increment 24 h, 109/L | 14 ± 14 (n = 953) | 8 ± 9 (n = 1007) | |
| CCI 24 h | 7 ± 7 (n = 953) | 4 ± 4 (n = 1007) | <.001 |
| Transfusion failure rate, median (IQR) | |||
| CCI 1 h, <7.5 | 0 (0-0.08) | 0.50 (0.09-0.75) | <.001 |
| CCI 24 h, <4.5 | 0 (0-0.33) | 0.50 (0.20-0.83) | <.001 |
| CCI 24 h, ≤0 | 0 (0-0) | 0 (0-0.08) | .013 |
| Per-protocol analysis | |||
| Platelet transfusions, n | 1395 | 1391 | |
| Efficacy parameters, mean ± SD | |||
| Count increment 1 h, 109/L | 25 ± 14 (n = 796) | 12 ± 8 (n = 868) | |
| CCI 1 h | 13 ± 7 (n = 796) | 7 ± 4 (n = 868) | <.001 |
| Count increment 24 h, 109/L | 14 ± 14 (n = 895) | 7 ± 8 (n = 897) | |
| CCI 24 h | 8 ± 7 (n = 895) | 4 ± 4 (n = 897) | <.001 |
| Transfusion failure rate, median (IQR) | |||
| CCI 1 h, <7.5 | 0 (0-0.02) | 0.50 (0.16-0.89) | <.001 |
| CCI 24 h, <4.5 | 0 (0-0.33) | 0.50 (0.18-0.93) | <.001 |
| CCI 24 h, ≤0 | 0 (0-0) | 0 (0-0.02) | .014 |
IQR, interquartile range.
Transfusion requirement
| . | Control . | Intervention . | P . |
|---|---|---|---|
| ITT analysis | |||
| Transfusion-treatment periods, n | 279 | 277 | |
| Red cell transfusions, median (IQR), n | 4 (2-7) | 4 (2-6) | .135 |
| Plasma transfusions, median (IQR), n | 0 (0-0) | 0 (0-0) | .842 |
| PLT transfusion interval, mean (95% CI), h* | 83 (77-91) | 71 (67-77) | .002 |
| PLT transfusions per transfusion-treatment period, median (IQR), n | 4 (2-7) | 5 (2.5-7.5) | .328 |
| Per-protocol analysis | |||
| Transfusion-treatment periods, n | 220 | 205 | |
| Red cell transfusions, median (IQR), n | 3 (2-6) | 3 (2-5) | .34 |
| Plasma transfusions, median (IQR), n | 0 (0-0) | 0 (0-0) | .59 |
| PLT transfusion interval, mean (95% CI), h* | 91 (83-100) | 71 (67-77) | <.001 |
| PLT transfusions per transfusion-treatment period, median (IQR), n | 3 (2-6.75) | 5 (3-7.5) | .085 |
| . | Control . | Intervention . | P . |
|---|---|---|---|
| ITT analysis | |||
| Transfusion-treatment periods, n | 279 | 277 | |
| Red cell transfusions, median (IQR), n | 4 (2-7) | 4 (2-6) | .135 |
| Plasma transfusions, median (IQR), n | 0 (0-0) | 0 (0-0) | .842 |
| PLT transfusion interval, mean (95% CI), h* | 83 (77-91) | 71 (67-77) | .002 |
| PLT transfusions per transfusion-treatment period, median (IQR), n | 4 (2-7) | 5 (2.5-7.5) | .328 |
| Per-protocol analysis | |||
| Transfusion-treatment periods, n | 220 | 205 | |
| Red cell transfusions, median (IQR), n | 3 (2-6) | 3 (2-5) | .34 |
| Plasma transfusions, median (IQR), n | 0 (0-0) | 0 (0-0) | .59 |
| PLT transfusion interval, mean (95% CI), h* | 91 (83-100) | 71 (67-77) | <.001 |
| PLT transfusions per transfusion-treatment period, median (IQR), n | 3 (2-6.75) | 5 (3-7.5) | .085 |
IQR, interquartile range; PLT, platelet.
Using all treatment periods via mixed Poisson model.
Safety: infections and (severe) adverse events, including transfusion reactions
There was a considerable number of infectious complications, adverse events, and serious adverse events (SAEs), without differences between the 2 study arms (supplemental Table 5). In both arms, 1 SAE was related to the platelet transfusion, an anaphylactic transfusion reaction to an off-protocol transfusion of platelets in additive solution in the control arm, and a transfusion-associated lung injury in the intervention arm (imputability possible). The percentage of transfusion reactions with imputability probable, possible, or certain was 2.8% in the control arm and 3.3% in the intervention arm. The majority of the transfusion reactions in both arms resulted in no or only minor morbidity.
Alloimmunization
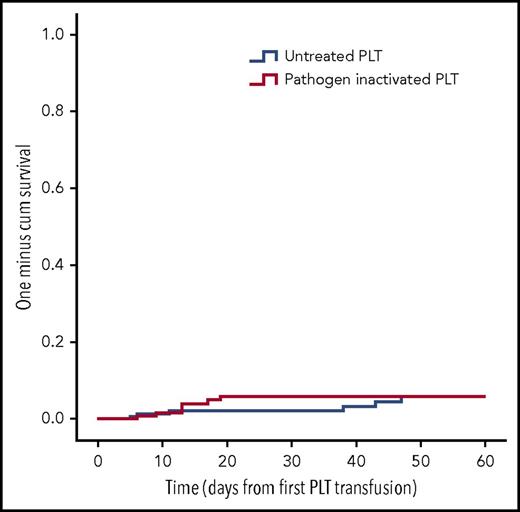
For the alloimmunization, we only included the first randomization transfusion-treatment periods of patients (n = 463). Excluding treatment periods with 0 or only 1 collected sample, as well as patients with HLA antibodies at the onset of their transfusion-treatment period, resulted in 356 evaluable treatment periods in the per-protocol–only population (control, n = 177; intervention, n = 179). As shown in Figure 3 the number of patients developing HLA class I alloantibodies was similar: 6 in the control arm and 7 in the intervention arm (risk ratio, 1.00; 95% CI, 0.34-2.98; P = 1.00). The ITT and per-protocol analyses are shown in supplemental Figures 2 and 3.
Kaplan-Meier analysis HLA class I alloimmunization. Time to the appearance of HLA class I alloantibodies in the per-protocol–only population (ie, a signal >5 SD above the normalized background signal in the Luminex assay). PLT, platelet.
Kaplan-Meier analysis HLA class I alloimmunization. Time to the appearance of HLA class I alloantibodies in the per-protocol–only population (ie, a signal >5 SD above the normalized background signal in the Luminex assay). PLT, platelet.
Discussion
Using WHO bleeding as a primary outcome, we compared pathogen-inactivated platelet products using riboflavin and UV light with standard plasma-stored platelet products in a multicenter international randomized controlled trial using a noninferiority design. The percentages of bleeding patients is on the same order of magnitude as other large randomized platelet-transfusion trials, although somewhat higher compared with the other 2 trials testing riboflavin/UV light–treated platelets, indicating that bleeding symptoms were accurately captured in the participating sites.10,11,17,18 Although the noninferiority criterion was met in the ITT analysis, the per-protocol analysis showed a slight increase in grade ≥2 bleeding complications in the intervention arm, as the upper limit of the 95% CI of the difference crossed the margin of 12.5 percentage points. As has been recently discussed by Mauri and D’Agostino,21 ITT and per-protocol analyses have important merits, as well as pitfalls, in noninferiority trials. Reporting both is considered the standard, with similar results in both supporting the robustness of the findings.21 In the ITT analysis in our study, off-protocol transfusions, as well as the inclusion of bleeding complications occurring between randomization and the first on-study platelet transfusion, likely resulted in a diluting effect to the advantage of the intervention arm. However, the per-protocol analysis might be hampered by selection bias. It is conceivable that excluding patients with active bleeding at the day of the first on-study transfusion resulted in a bias to the advantage of the control arm by removing patients with a bleeding tendency. A modified per-protocol population analysis, not excluding patients with active bleeding, reduced the difference with regard to bleeding complications between both populations slightly, although still not meeting the noninferiority criterion.
With regard to secondary bleeding end points, there were no differences between the 2 study arms. Importantly, although the numbers are small, no differences were observed with regard to severe bleeding complications, pertinent to daily clinical practice. There were no differences with regard to the consumption of red cell concentrates or plasma, considered to be surrogate markers for clinically significant bleeding complications.
The small detrimental effect on hemostasis seen in the per-protocol analysis is in concordance with the conclusions of the most recent Cochrane analysis on pathogen reduction, as well as the outcome of the recently published Evaluation of the Efficacy of Platelets Treated With Pathogen Reduction Process study, which compared amotosalen and UV-A–treated platelets with platelets in plasma, as well as platelet additive solution.8,22 The observed increase in bleeding complications is likely due to the detrimental effects on platelet function induced by pathogen reduction, as has been shown in vitro for all of the currently available pathogen-reduction techniques.23,24
All transfusion-increment parameters were in favor of the control arm, which translated to a higher usage of platelet products in the intervention arm because the transfusion trigger is met sooner, with an increase ∼1 product per patient. This is as expected, because recently published clinical studies comparing pathogen-reduced platelet concentrates with untreated platelets also report higher platelet transfusion need.11,22 Possibly, the lower CCIs are also due to the effects on platelets induced by pathogen inactivation, which were described for several pathogen-inactivation methods.22,25,26 This subject should be the basis for future research.
As expected in this population, there was a high number of adverse events and SAEs, with only 2 SAEs related to platelet transfusion. In the intervention arm, a possible transfusion-related acute lung injury was reported. All platelet products in plasma can cause a transfusion-related acute lung injury; because pathogen-inactivation does not target proteins, such an occurrence is not unexpected. In contrast to recently published animal studies, pathogen-inactivation treatment did not result in a reduction in HLA class I alloimmunization.7,27 Because the percentage of immunized patients is low in both arms, this result may be completely explained by randomness. Additionally, the discrepancy between animal and human studies may be explained by the administration of untreated red blood cells in patients in both arms, which did not occur in the animal experiments. The recently published data of the Italian Platelet Technology Assessment Study trial also reported comparable low rates of HLA class I antibodies.28
The numbers of countries, hospitals, and patients, as well as the large numbers of platelet transfusions and observed days, are the main strengths of this study, contributing to the generalizability of conclusions regarding the clinical efficacy of pathogen-inactivated buffy coat platelets in thrombocytopenic hematology patients. Despite efforts to reduce this, the main weakness of our study is the significant number of patients with off-protocol transfusions. Because our study has shown a mildly reduced hemostatic efficacy, as well as a significant impact on transfusion increments, whether to implement pathogen-inactivated platelet products really depends on the balance between increased safety for known and unknown pathogens, which varies among countries worldwide, and the clinical effects that pathogen inactivation have on the platelet product. Health-economic arguments should also be taken into account. Clearly, there is room and a need to improve the current techniques of platelet pathogen inactivation. Indeed, replacing plasma with novel additive solutions has recently shown promising results.29 Moreover, a clinical trial using pathogen inactivation in apheresis platelets, potentially contributing to a decreased risk in alloimmunization, is about to start.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all data managers, particularly Julie Carruthers and Erin Jamula, for their efforts as central data managers and center coordinators for the Canadian centers. They also thank Stephanie Willing, Kate Kelly, Shawna Reddie, and Geetha Yogendran for their site coordinatorship in the Canadian centers. In addition, the authors thank auditors, nursing staff in all participating hospitals. The authors also thank Rutger Middelburg and John Matthews for their acquisition of data and Rutger Middelburg for his role in the electronic adjudication of bleeding. They thank Henk W. Reesink (chairman DSMB), Ronald Brand (member DSMB), Jeroen C.J. Eikenboom (member DSMB), and Donald Arnold (member DSMB) for safety oversight. For help with the statistical analysis plan, the authors thank Richard Cook, Richard Weiskopf, and Theo Stijnen. For assistance with the collection, processing, quality monitoring, and distribution of platelet products, the authors wish to thank all blood bank personnel from Sanquin Blood Bank (Amsterdam, The Netherlands), Haukeland University Blood Bank (Bergen, Norway), and Canadian Blood Services (Ottawa, ON, Canada) and extend special thanks to Guido Kruit and Chantal Couture. They thank (local) hospital blood banks and transfusion laboratories, without whom this study would not have been completed. The authors are most grateful to all patients who agreed to participate in the PREPAReS trial. They authors wish to thank Terumo BCT for their support.
This work was supported by research funding from Terumo BCT.
The funding body had no role in data collection, study management, or analyses.
Authorship
Contribution: P.F.v.d.M., J.A.v.H., R.J.v.W.-V., R.P.G., A.B., and J.-L.H.K. contributed to the conception and design of the study. P.F.Y., R.J.v.W.-V., O.E., J.J.Z., M.T., E.A.M.B., P.t.B., A.T., Y.L., C.H., D.L., P.J.N., T.H., N.M.H., and J.-L.H.K. contributed to the acquisition of data. P.F.v.d.M., P.F.Y., N.v.G., and J.-L.H.K. analyzed and interpreted the data and wrote the manuscript. R.J.v.W.-V., R.P.G., A.B., and J.G.v.d.B. contributed to the interpretation of the data. All authors revised the manuscript critically for content, gave final approval for the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: P.F.Y. reports department honoraria for serving as a member of the advisory board for Genzyme, N.v.G. reports departmental honoraria for serving on the scientific advisory board for CSL Behring. P.t.B. received a consultancy fee from Novartis and speakers’ bureau fees from Novartis, Shire, and Celgene. Y.L. received consultancy fees from Amgen and Pfizer and research funding from Novartis and Octapharma. C.H. serves as a consultant for and received speakers’ bureau fees from Abbvie, Amgen, Celgene, Janssen, and Novartis. P.J.N. received research funding from Terumo BCT and Cerus. R.P.G. received consultancy fees from Terumo BCT and is a member of the AABB Public Policy and Strategy Committee (nonprofit), the Advisory Board of the School of Biomedical Engineering at Colorado State University, and the Advisory Board for the Northern Colorado BioScience Association (nonprofit organization). T.H. reports research funding from the US Department of Defense and Terumo BCT. N.M.H. is a member of the Scientific Research Advisory Committee, Canadian Blood Services. The remaining authors declare no competing financial interests.
Correspondence: Pieter F. van der Meer, Center for Clinical Transfusion Research-Sanquin, Plesmanlaan 1A, 2333BZ, Leiden, The Netherlands; e-mail: p.vandermeer@sanquin.nl.
REFERENCES
Author notes
P.F.v.d.M. and P.F.Y. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal