Key Points
Children with acute leukemia given αβhaplo-HSCT have a lower risk for acute and chronic GVHD than those transplanted from an unrelated donor.
GVHD-free, relapse-free survival after αβhaplo-HSCT is better than that observed in unrelated donor HSCT recipients.
Abstract
Traditionally, hematopoietic stem cell transplantation (HSCT) from both HLA-matched related and unrelated donors (UD) has been used for treating children with acute leukemia (AL) in need of an allograft. Recently, HLA-haploidentical HSCT after αβ T-cell/B-cell depletion (αβhaplo-HSCT) was shown to be effective in single-center studies. Here, we report the first multicenter retrospective analysis of 127 matched UD (MUD), 118 mismatched UD (MMUD), and 98 αβhaplo-HSCT recipients, transplanted between 2010 and 2015, in 13 Italian centers. All these AL children were transplanted in morphological remission after a myeloablative conditioning regimen. Graft failure occurred in 2% each of UD-HSCT and αβhaplo-HSCT groups. In MUD vs MMUD-HSCT recipients, the cumulative incidence of grade II to IV and grade III to IV acute graft-versus-host disease (GVHD) was 35% vs 44% and 6% vs 18%, respectively, compared with 16% and 0% in αβhaplo-HSCT recipients (P < .001). Children treated with αβhaplo-HSCT also had a significantly lower incidence of overall and extensive chronic GVHD (P < .01). Eight (6%) MUD, 32 (28%) MMUD, and 9 (9%) αβhaplo-HSCT patients died of transplant-related complications. With a median follow-up of 3.3 years, the 5-year probability of leukemia-free survival in the 3 groups was 67%, 55%, and 62%, respectively. In the 3 groups, chronic GVHD-free/relapse-free (GRFS) probability of survival was 61%, 34%, and 58%, respectively (P < .001). When compared with patients given MMUD-HSCT, αβhaplo-HSCT recipients had a lower cumulative incidence of nonrelapse mortality and a better GRFS (P < .001). These data indicate that αβhaplo-HSCT is a suitable therapeutic option for children with AL in need of transplantation, especially when an allele-matched UD is not available.
Introduction
During the last 4 decades, allogeneic hematopoietic stem cell transplantation (HSCT) from an HLA-matched donor, either related or unrelated (UD), has been extensively used to treat patients with both malignant and nonmalignant disorders.1 However, only 25% of patients who are candidates to receive allogeneic HSCT have an HLA-identical sibling, and a suitable UD can be identified for less than 60% of the remaining patients.2 The likelihood of finding an optimal donor varies among racial and ethnic groups, with the probability of identifying an appropriate donor being highest among whites of European descent (75%) and lowest among blacks of South or Central American descent (16%).3 In the absence of an HLA-matched donor, alternative donor/sources of HSCs, such as unrelated umbilical cord blood and HLA-haploidentical relatives, are being increasingly used.2,4,5 The majority of patients have a family member with 1 identical HLA-haplotype and the other mismatched (ie, haploidentical).6 Although mature donor T cells present in the graft facilitate T-cell reconstitution, they are also responsible for the occurrence of graft-versus-host disease (GVHD).7,8 Different strategies, based on either pharmacological immunosuppression or T-cell depletion of the graft, have been developed to prevent GVHD after HLA-haploidentical HSCT.9-12 Pioneering studies in adults have demonstrated that the infusion of megadoses of purified CD34+ cells can prevent both graft rejection and severe GVHD in haplo-HSCT recipients.13,14 However, extensive lymphoid cell depletion has resulted in an increased risk for opportunistic infections, especially in the first months after HSCT. In the attempt to reduce the risk for infections, recently, a new strategy of graft manipulation has been implemented, based on the selective elimination of αβ T cells and CD19+ B cells (αβhaplo-HSCT).15 This refined technique of graft engineering reduces the problems associated with delayed immune recovery, which is typical in the CD34+ cell selection haplo-HSCT approach. Indeed, using αβhaplo-HSCT, it is possible to transfer to the recipient not only donor HSCs but also committed hematopoietic progenitors, as well as mature natural killer (NK) and γδ T cells.16,17 These lymphocyte subsets may provide a protective effect against both leukemia relapse and severe infections.18-21 Using αβhaplo-HSCT, we reported promising clinical results in children with life-threatening nonmalignant disorders.22 More recently, single-center experiences in pediatric patients with malignancies have been published, showing the risks for nonrelapse mortality (NRM) and leukemia relapse being comparable to those from HLA-identical siblings or UD-HSCT.23-25 Moreover, patients receiving αβhaplo-HSCT had a lower risk for both acute and chronic GVHD, leading to better GVHD-free/relapse-free survival (GRFS).25
Here, we report the first multicenter, retrospective comparative analysis on the outcome of children with acute leukemia (AL) given either UD-HSCT or αβhaplo-HSCT. Using data reported by 13 centers affiliated with the Associazione Italiana di Oncoematologia Pediatrica-HSCT network, we evaluated children undergoing a first HSCT between October 2010 and December 2015. These data, obtained in a multicenter setting, show that the outcome of children given αβhaplo-HSCT is equivalent to that of patients transplanted from an 8/8 UD-HSCT (MUD), whereas it is superior to that of patients given an allograft from a HLA-mismatched UD-HSCT (7/8 or 6/8, mismatched MUD [MMUD]).
Patients and methods
This is a retrospective, registry-based analysis conducted on behalf of the Associazione Italiana di Oncoematologia Pediatrica-HSCT network. Table 1 summarizes main patient, donor, and transplant characteristics. Six centers performed both UD-HSCT and αβhaplo-HSCT, whereas in the remaining 7 centers, only the former procedure was performed. Seventy of the patients enrolled in the αβhaplo-HSCT group have been already published in a recent single-center experience.25 All consecutive patients younger than 21 years with either acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) who received a first allograft in morphological complete remission after a myeloablative conditioning from an UD or αβhaplo-HSCT between October 2010 and December 2015 were enrolled. Written informed consent was obtained from either the patient or parents/legal guardians in accordance with the Helsinki Declaration, and the study was approved by the local Ethical Committee. The UD was selected using high-resolution typing for the HLA-loci A, B, C, and DRB1. A MUD was employed in 52% of cases; the remaining 48% of the patients were transplanted from a donor with either 1 or 2 HLA disparities (MMUD). Centers performing αβhaplo-HSCT offered this approach to children lacking either an HLA-identical sibling or a MUD, or in need of an urgent procedure, according to physician’s judgment. Total body irradiation (TBI) was used in patients older than 3 years affected by either ALL or very high risk AML (ie, those with cytogenetic/molecular features predicting high risk for relapse; see Table 1 for further details). Anti-T-lymphocyte globulin (ATLG [Grafalon; Neovii Biotech] or antithymocyte globulin [Thymoglobulin; Sanofi-Genzyme]) was administered to all patients for preventing both graft rejection and GVHD. Moreover, in the αβhaplo-HSCT group, for further lowering the risk for Epstein-Barr virus (EBV)-related posttransplant lymphoproliferative disorder (PTLD), on day −1, patients were given rituximab (200 mg/m2) for in vivo depletion of both donor and recipient B cells. No patient in the αβhaplo-HSCT group received posttransplant pharmacological GVHD prophylaxis, whereas the post-HSCT combination of calcineurin inhibitor and short-term methotrexate was employed for preventing GVHD occurrence in UD-HSCT recipients (see Table 1).
Patient, donor, and transplant characteristics
| . | MUD . | MMUD . | Haploidentical . | P . |
|---|---|---|---|---|
| Number of patients | 127 | 118 | 98 | |
| Sex | ||||
| Male | 74 (58%) | 71 (60%) | 65 (66%) | .450 |
| Female | 53 (42%) | 47 (40%) | 33 (34%) | |
| Median age at diagnosis (range), y | 5.7 (0.2-17.4) | 8.8 (0.3-17.5) | 6.6 (0.1-17.3) | .113 |
| Diagnosis | ||||
| ALL | 84 (66%) | 86 (73%) | 68 (68%) | .513 |
| AML | 43 (34%) | 32 (27%) | 30 (32%) | |
| ALL phenotype | ||||
| B-cell precursor | 71 (85%) | 67 (78%) | 53 (78%) | .474 |
| T-cell precursor | 13 (15%) | 19 (22%) | 15 (22%) | |
| AML FAB classification | ||||
| AML M0 | 1 (2%) | 2 (6%) | 3 (10%) | .623 |
| AML M1 | 7 (17%) | 6 (19%) | 4 (13%) | |
| AML M2 | 13 (30%) | 4 (13%) | 6 (20%) | |
| AML M3 | 1 (2%) | 1 (3%) | 1 (3%) | |
| AML M4 | 5 (12%) | 4 (13%) | 5 (17%) | |
| AML M5 | 12 (28%) | 13 (40%) | 8 (27%) | |
| AML M6 | 3 (7%) | 1 (3%) | 0 (0%) | |
| AML M7 | 1 (2%) | 1 (3%) | 3 (10%) | |
| Disease phase at HSCT | ||||
| ALL | ||||
| CR1 | 35 (42%) | 39 (45%) | 19 (28%) | <.001 |
| CR2 S1-S2 | 35 (42%) | 28 (33%) | 22 (32%) | |
| CR2 S3-S4 | 14 (16%) | 18 (21%) | 17 (25%) | |
| Other CR | 0 | 1 (1%) | 10 (15%) | |
| AML | ||||
| CR1 | 33 (77%) | 26 (81%) | 23 (77%) | .803 |
| CR2 | 9 (21%) | 6 (19%) | 7 (23%) | |
| Other CR | 1 (2%) | 0 (0%) | 0 (0%) | |
| Median age at HSCT (range), y | 5.7 (1.0-18.0) | 10.3 (0.4-18.1) | 9.4 (0.9-18.2) | .106 |
| Stem cell source | ||||
| BM | 97 (76%) | 90 (76%) | 0 (0%) | <.001 |
| PBSC | 30 (24%) | 28 (24%) | 98 (100%) | |
| Conditioning regimen | ||||
| TBI-based | 64 (50%) | 68 (58%) | 72 (74%) | .016 |
| Busulfan-based | 54 (43%) | 43 (36%) | 18 (18%) | |
| Treosulfan-based | 8 (6%) | 6 (5%) | 7 (7%) | |
| Other chemotherapy | 1 (1%) | 1(1%) | 1 (1%) | |
| GVHD prophylaxis | ||||
| Cs-A+MTX | 2 (2%) | 2 (2%) | 0 (0%) | <.001 |
| Cs-A+MTX+ATLG | 112 (89%) | 97 (82%) | 0 (0%) | |
| Cs-A+MTX+ATLG+MMF | 0 (0%) | 6 (5%) | 0 (0%) | |
| Tacrolimus+MTX+ATLG | 12 (9%) | 13 (11%) | 0 (0%) | |
| αβ+ and CD19+ negative selection + ATLG | 0 (0%) | 0 (0%) | 98 (100%) | |
| Cell dose infused | ||||
| Total nucleated cell dose (range), ×108/kg | 5.5 (1.7-48) | 5.4 (2.2-43) | 10.2 (3-52) | <.001 |
| CD34+ cells, ×106/kg | 5.2 (1.0-40) | 4.9 (2.1-39) | 14.4 (5.5-56) | <.001 |
| CD3+ αβ+ T lymphocytes, ×106/kg | — | — | 0.04 (0.00-0.99) | — |
| CD3+ γδ+ T lymphocytes, ×106/kg | — | — | 8.1 (1.0-64.6) | — |
| . | MUD . | MMUD . | Haploidentical . | P . |
|---|---|---|---|---|
| Number of patients | 127 | 118 | 98 | |
| Sex | ||||
| Male | 74 (58%) | 71 (60%) | 65 (66%) | .450 |
| Female | 53 (42%) | 47 (40%) | 33 (34%) | |
| Median age at diagnosis (range), y | 5.7 (0.2-17.4) | 8.8 (0.3-17.5) | 6.6 (0.1-17.3) | .113 |
| Diagnosis | ||||
| ALL | 84 (66%) | 86 (73%) | 68 (68%) | .513 |
| AML | 43 (34%) | 32 (27%) | 30 (32%) | |
| ALL phenotype | ||||
| B-cell precursor | 71 (85%) | 67 (78%) | 53 (78%) | .474 |
| T-cell precursor | 13 (15%) | 19 (22%) | 15 (22%) | |
| AML FAB classification | ||||
| AML M0 | 1 (2%) | 2 (6%) | 3 (10%) | .623 |
| AML M1 | 7 (17%) | 6 (19%) | 4 (13%) | |
| AML M2 | 13 (30%) | 4 (13%) | 6 (20%) | |
| AML M3 | 1 (2%) | 1 (3%) | 1 (3%) | |
| AML M4 | 5 (12%) | 4 (13%) | 5 (17%) | |
| AML M5 | 12 (28%) | 13 (40%) | 8 (27%) | |
| AML M6 | 3 (7%) | 1 (3%) | 0 (0%) | |
| AML M7 | 1 (2%) | 1 (3%) | 3 (10%) | |
| Disease phase at HSCT | ||||
| ALL | ||||
| CR1 | 35 (42%) | 39 (45%) | 19 (28%) | <.001 |
| CR2 S1-S2 | 35 (42%) | 28 (33%) | 22 (32%) | |
| CR2 S3-S4 | 14 (16%) | 18 (21%) | 17 (25%) | |
| Other CR | 0 | 1 (1%) | 10 (15%) | |
| AML | ||||
| CR1 | 33 (77%) | 26 (81%) | 23 (77%) | .803 |
| CR2 | 9 (21%) | 6 (19%) | 7 (23%) | |
| Other CR | 1 (2%) | 0 (0%) | 0 (0%) | |
| Median age at HSCT (range), y | 5.7 (1.0-18.0) | 10.3 (0.4-18.1) | 9.4 (0.9-18.2) | .106 |
| Stem cell source | ||||
| BM | 97 (76%) | 90 (76%) | 0 (0%) | <.001 |
| PBSC | 30 (24%) | 28 (24%) | 98 (100%) | |
| Conditioning regimen | ||||
| TBI-based | 64 (50%) | 68 (58%) | 72 (74%) | .016 |
| Busulfan-based | 54 (43%) | 43 (36%) | 18 (18%) | |
| Treosulfan-based | 8 (6%) | 6 (5%) | 7 (7%) | |
| Other chemotherapy | 1 (1%) | 1(1%) | 1 (1%) | |
| GVHD prophylaxis | ||||
| Cs-A+MTX | 2 (2%) | 2 (2%) | 0 (0%) | <.001 |
| Cs-A+MTX+ATLG | 112 (89%) | 97 (82%) | 0 (0%) | |
| Cs-A+MTX+ATLG+MMF | 0 (0%) | 6 (5%) | 0 (0%) | |
| Tacrolimus+MTX+ATLG | 12 (9%) | 13 (11%) | 0 (0%) | |
| αβ+ and CD19+ negative selection + ATLG | 0 (0%) | 0 (0%) | 98 (100%) | |
| Cell dose infused | ||||
| Total nucleated cell dose (range), ×108/kg | 5.5 (1.7-48) | 5.4 (2.2-43) | 10.2 (3-52) | <.001 |
| CD34+ cells, ×106/kg | 5.2 (1.0-40) | 4.9 (2.1-39) | 14.4 (5.5-56) | <.001 |
| CD3+ αβ+ T lymphocytes, ×106/kg | — | — | 0.04 (0.00-0.99) | — |
| CD3+ γδ+ T lymphocytes, ×106/kg | — | — | 8.1 (1.0-64.6) | — |
Data are expressed as median and range or as absolute number and column percentage, as appropriate. Indications for hematopoietic stem cell transplantation in CR1 of patients with ALL were high level of minimal residual disease at end of induction therapy (ie, >1 × 10−3 at day +78 after beginning of treatment), high-risk infant ALL, t(4;11), hypodiploidy (≤43 chromosomes), and hyperleukocytosis T ALL with poor response to the steroid prephase. Indications for hematopoietic stem cell transplantation in CR1 of patients with AML were t(10;11), t(6;11), t(6;9), t(5;11), complex karyotype (≥3 either numeric or structural aberrations), FLT3-ITD with high allelic ratio, M7 AML, CBFA2T3-GLIS2 fusion transcript, and not being in morphological CR after the first of the 2 induction courses.
BM, bone marrow; CR, complete remission; CsA, cyclosporine-A; MMF, mycophenolate mofetil; MTX, methotrexate; PBSC, peripheral blood stem cells.
Minimal residual disease (MRD) evaluated by multicolor flow cytometry within 30 days before HSCT was available in 163 patients (47.8% of the overall cohort). Among these patients, it was less than 1 × 10−3 in 143 (88%) and more than 1 × 10−3 in 20 (12%) cases.
Within the αβhaplo-HSCT group, the donor was the mother for 58 patients (59%) and the father for the remaining 40 patients (41%). The HLA-haploidentical donor was selected according to immunological criteria, giving priority to NK alloreactivity (evaluated through the killer immunoglobulin-like receptor [KIR]/ligand model),26,27 KIR B haplotype,28 higher B-content score,29 and size of NK alloreactive subset.30-32 An NK alloreactive donor was employed in 41 (43%) of 96 evaluable donor/recipient pairs.
αβhaplo-HSCT donors received granulocyte-colony stimulating factor for 4 days at the total dose of 12 µg/kg body weight, and apheresis was performed on the fifth day. When on day 4 the CD34+ cell count was less than 40/µL and/or the predicted apheresis yields was less than 12.0 × 106 CD34+ HSCs/kg recipient’s body weight, a CXCR4 antagonist (Plerixafor, Mozobil) was given for boosting mobilization of HSCs/progenitor cells.33,34 In all centers, manipulations were performed in a closed system. Clinical-grade reagents, disposable kits, and instrumentation were from Miltenyi Biotec (Bergisch Gladbach, Germany).
In the UD-HSCT group, the median number of total nucleated cells infused was 5.6 × 108/kg, whereas the median number of CD34+ infused was 5 × 106/kg. Children enrolled in the αβhaplo-HSCT cohort received a median number of 14.4 × 106/kg CD34+ cells. In this latter case, the αβ T-cell depletion was homogeneously very efficient, the median number of residual αβ T cells infused into the graft being 0.04 × 106/kg. As previously reported, a large number of NK and γδ T cells was retained into the graft (see Table 1 for further details).34
Chimerism analysis evaluated through the Short Tandem Repeats polymorphism was performed on cells obtained from bone marrow aspirates whenever performed, and on peripheral mononuclear cells, weekly for the first 3 months and monthly thereafter.
Definitions and statistical analysis
Graft failure was defined as either lack of initial engraftment of donor cells or loss of donor cells after initial engraftment. Time to neutrophil engraftment was defined as time from HSCT to the first of 3 consecutive days with an absolute neutrophil count of at least 0.5 × 109/L, whereas time to platelet engraftment was defined as time from HSCT to the first of 7 consecutive days with an unsupported platelet count of at least 50 × 109/L.
Results are reported in the 2 UD and αβhaplo groups. Moreover, the effect of the type of donor on the clinical outcome are assessed in 3 different groups: 8/8 UD-HSCT (MUD), 6/8 or 7/8 UD-HSCT (MMUD), and αβhaplo-HSCT (supplemental Table 1, available on the Blood Web site).
Patients surviving more than 14 and 100 days after HSCT were evaluated for acute and chronic GVHD, respectively, which were diagnosed and graded according to previously published criteria.35,36 NRM was defined as the probability of death from any cause other than recurrence of leukemia. Overall survival (OS) was defined as the probability of being alive at last follow-up, whereas leukemia-free survival (LFS) was defined as the probability of survival, without evidence of disease at any time after HSCT. In estimating LFS, death and relapse were considered events, whereas patients who were alive, with sustained donor engraftment and disease-free, were censored at last follow-up. We also evaluated the composite end point of chronic GRFS.37
Data on patients transplanted from either an HLA-haploidentical relative or a UD were collected in the data warehouse of the Associazione Italiana di Oncoematologia Pediatrica-HSCT group. Both primary and secondary end points were assessed by the statistician of the group (M.Z.). Data were analyzed as of 31 December 2017. Quantitative variables were reported as median value and range, whereas categorical variables were expressed as absolute value and percentage. Demographic and clinical characteristics of the patients were compared using the χ-square test or Fisher’s exact test for categorical variables, whereas the Mann-Whitney rank sum test or the Student t-test were used for continuous variables, whenever appropriate. Acute and chronic GVHD, rejection, engraftment, OS, LFS, NRM, and relapse incidence were estimated from the date of HSCT to the date of an event or last follow-up. Probabilities of LFS and OS were calculated according to the Kaplan and Meier method.38 Acute GVHD and chronic GVHD, NRM, and relapse were calculated as cumulative incidence curves to adjust the estimates for competing risks. All results were expressed as either probability or cumulative incidence (%) with 95% confidence interval (CI).39,40
The significance of differences between LFS and OS was estimated by the log-rank test (Mantel-Cox), whereas Gray’s test was used to assess, in univariable analyses, differences between cumulative incidences.41
Multivariable analysis was performed using the Cox proportional hazard regression model or the method of Fine and Gray, as appropriate.39,40 All variables significant in univariable analysis were included in the multivariable model.
P values <.05 were considered statistically significant. Statistical analyses were performed by means of NCSS (NCSS 10 Statistical Software, https://www.ncss.com/software/ncss/) and Stata MP/15 (StataCorp LP, http://www.stata.com/).
Results
Engraftment and immune recovery
In the entire cohort (343 patients), 6 patients did not engraft: 2 each in the MUD (1.6%), MMUD (1.7%), and αβhaplo-HSCT (2%) groups. All children experiencing primary graft failure in the UD-HSCT group had received less than 4 × 106 CD34+/kg, and 2 of them received less than 2 × 106 CD34+/kg. Three of these patients died after rejecting the graft because of infectious complications, and the remaining patient relapsed. No correlation between graft composition and graft failure was observed in the αβhaplo-HSCT cohort. One of the 2 patients who experienced graft failure in this latter group was successfully retransplanted using the other parent as a donor. The second patient died of a disseminated adenovirus infection. Patients given αβhaplo-HSCT had a faster neutrophil and platelet recovery than those transplanted from a MUD or a MMUD (P < .001; Figure 1A-B, respectively). Moreover, MMUD-HSCT recipients had a lower probability of platelet recovery at 100 days after the allograft in comparison with patients receiving either αβhaplo or MUD-HSCT (Figure 1B). Notably, in the αβhaplo-HSCT group, no patient received posttransplant granulocyte-colony stimulating factor to accelerate neutrophil recovery.
Engraftment's kinetic and immune recovery in αβhaplo-HSCT, MUD-HSCT, and MMUD-HSCT recipients. Cumulative incidence of neutrophil (A) and platelet engraftment (B), recovery of CD3+ cells (C), CD3+/CD4+ cells (D), CD3+/CD8+ cells (E), and B cells (F), according to the type of donor employed.
Engraftment's kinetic and immune recovery in αβhaplo-HSCT, MUD-HSCT, and MMUD-HSCT recipients. Cumulative incidence of neutrophil (A) and platelet engraftment (B), recovery of CD3+ cells (C), CD3+/CD4+ cells (D), CD3+/CD8+ cells (E), and B cells (F), according to the type of donor employed.
Data on immune reconstitution were available in 94 (96%), 95 (75%), and 90 (76%) αβhaplo, MUD, and MMUD-HSCT recipients. Recovery of CD3+ and of CD3+/CD8+ cells was faster in patients given MUD and MMUD-HSCT in comparison with that of patients given αβhaplo-HSCT until 6 months after the allograft (Figure 1C,E, respectively). Reconstitution of CD3+/CD4+ was better in the MUD and MMUD groups only at 3 months, whereas at 12 months it was better in αβhaplo-HSCT than in the other 2 groups. B-cell recovery mimicked that of CD3+ and CD3+/CD8+ cells (Figure 1F).
Acute and chronic GVHD
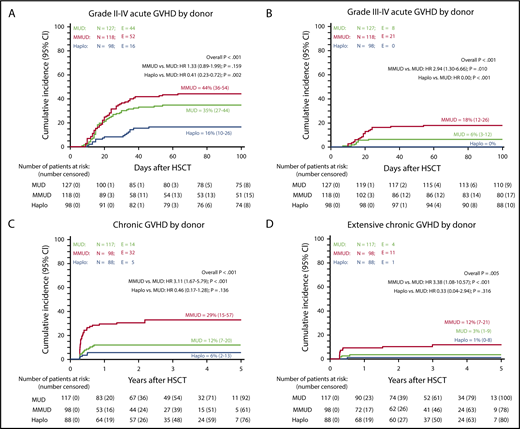
The cumulative incidence of grade II to IV and grade III to IV acute GVHD in patients given MUD vs MMUD-HSCT was 35% vs 44% and 6% vs 18%, respectively, as compared with 16% and 0% in αβhaplo-HSCT recipients (P < .001; Figure 2A-B). Remarkably, no patients enrolled in this latter group developed visceral involvement of acute GVHD. Children treated with αβhaplo-HSCT benefited also from a lower incidence of both overall and extensive chronic GVHD. Indeed, although the cumulative incidence of overall and extensive chronic GVHD in patients given MUD vs MMUD-HSCT was 12% vs 29% and 3% vs 12%, respectively, in recipients of αβhaplo-HSCT, it was 6% and 1% (P < .001 and P < .005, respectively; Figure 2C-D; see supplemental Table 1 for more details). The only patient experiencing extensive chronic GVHD in the αβhaplo-HSCT group received a total number of αβ+ T cells equal to 0.025 × 106/kg.
Cumulative incidence of acute and chronic GVHD in αβhaplo-HSCT, MUD-HSCT, and MMUD-HSCT recipients. Cumulative incidence of grade II to IV acute GVHD (A), grade III to IV acute GVHD (B), chronic GVHD (C), and extensive chronic GVHD (D), according to the type of donor employed. All results were expressed as either probability or cumulative incidence (%) with 95% CI.
Cumulative incidence of acute and chronic GVHD in αβhaplo-HSCT, MUD-HSCT, and MMUD-HSCT recipients. Cumulative incidence of grade II to IV acute GVHD (A), grade III to IV acute GVHD (B), chronic GVHD (C), and extensive chronic GVHD (D), according to the type of donor employed. All results were expressed as either probability or cumulative incidence (%) with 95% CI.
Nonrelapse mortality and relapse
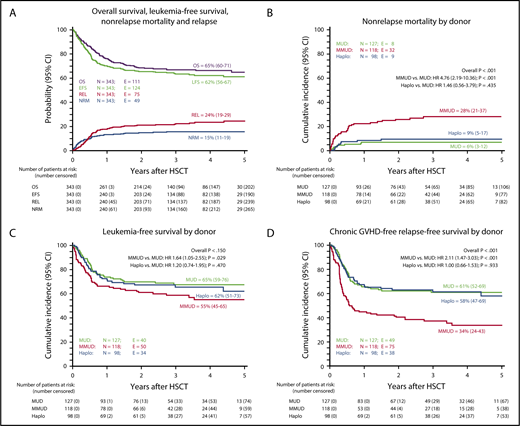
Forty-nine patients died of transplant-related complications (Figure 3A; Table 2): 40 in the UD-HSCT group (17%) and 9 who had received αβhaplo-HSCT (9%). Although the probability of NRM was superimposable in MUD-HSCT (6%) and αβhaplo-HSCT (9%), MMUD-HSCT was associated with a higher risk for mortality (28%; P < .001; Figure 3B), as confirmed in the multivariable analysis (hazard ratio [HR], 4.35; 95% CI, 1.97-9.60; P < .001), after adjusting for age, which was also associated with NRM (age, >13 years vs <13 years: HR, 1.88; 95% CI, 1.05-3.35; P = .033; Table 3). Overall, infection was the main cause of nonleukemia death, occurring in 4 (12%), 15 (33%), and 5 (16%) MUD, MMUD, and αβhaplo-HSCT recipients, respectively (supplemental Table 2). Although bacterial infections were significantly higher in MMUD recipients (34%) in comparison with MUD (17%) and αβhaplo-HSCT (8%), the incidence of fungal and viral infections was superimposable (P = N.S. (not significant); supplemental Table 3). Remarkably, none of the patients who received αβhaplo-HSCT developed EBV-related PTLD in comparison with 2 UD-HSCT recipients (1 each in the MUD and MMUD subgroup).
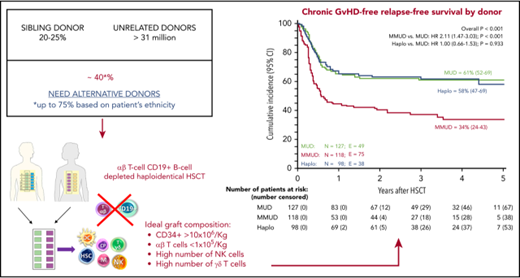
Probability of OS, LFS, NRM, relapse incidence (REL), and chronic GVHD-free, relapse-free survival (GFRS). OS, LFS, NRM, and REL of the overall cohort of patients included in the study (A). Cumulative incidence of NRM according to the type of donor employed (B). Five-year probability of LFS according to the type of donor employed (C). Five-year probability of GFRS according to the type of donor employed (D). All results were expressed as either probability or cumulative incidence (%) with 95% CI.
Probability of OS, LFS, NRM, relapse incidence (REL), and chronic GVHD-free, relapse-free survival (GFRS). OS, LFS, NRM, and REL of the overall cohort of patients included in the study (A). Cumulative incidence of NRM according to the type of donor employed (B). Five-year probability of LFS according to the type of donor employed (C). Five-year probability of GFRS according to the type of donor employed (D). All results were expressed as either probability or cumulative incidence (%) with 95% CI.
Univariate analysis of nonrelapse mortality in the cohort of patients analyzed
| . | Number of patients . | Events . | Cumulative incidence . | 95% CI . | P . | HR* . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|---|
| All patients | 343 | 49 | 15% | 11-19 | ||||
| Sex | ||||||||
| Male | 210 | 25 | 12% | 8-17 | .116 | |||
| Female | 133 | 24 | 18% | 13-27 | 1.57 | 0.90-2.74 | .116 | |
| Age at HSCT, y | ||||||||
| <9.5 | 172 | 15 | 9% | 6-15 | .003 | |||
| ≥9.5 | 171 | 34 | 20% | 15-27 | 2.42 | 1.32-4.45 | .004 | |
| <6 | 94 | 10 | 11% | 6-20 | .013 | |||
| 6-9.5 | 78 | 5 | 6% | 3-15 | 0.59 | 0.20-1.72 | .334 | |
| 9.5-13 | 80 | 13 | 16% | 10-27 | 1.59 | 0.69-3.65 | .274 | |
| ≥13 | 91 | 21 | 24% | 16-34 | 2.30 | 1.09-4.87 | .029 | |
| <13 | 252 | 28 | 13% | 8-16 | .006 | |||
| ≥13 | 91 | 21 | 24% | 16-34 | 2.95 | 1.25-3.84 | .006 | |
| Diagnosis | ||||||||
| ALL | 237 | 37 | 16% | 12-21 | .281 | |||
| AML | 106 | 12 | 12% | 7-20 | 0.79 | 0.37-1.34 | .280 | |
| ALL phenotype | ||||||||
| B-cell precursor | 191 | 29 | 16% | 11-22 | ||||
| T-cell precursor | 47 | 8 | 17% | 9-32 | .744 | 1.15 | 0.52-2.53 | .732 |
| Disease phase at HSCT | ||||||||
| Only ALL patients | ||||||||
| CR1† | 93 | 16 | 18% | 11-28 | .850 | |||
| CR2 S1-S2† | 85 | 11 | 13% | 8-23 | 0.72 | 0.34-1.56 | .405 | |
| CR2 S3-S4‡ | 49 | 8 | 16% | 9-31 | 0.93 | 0.40-2.17 | .865 | |
| Other CR‡ | 11 | 2 | 18% | 5-64 | 1.98 | 0.24-4.83 | .920 | |
| Only AML patients | ||||||||
| CR1† | 82 | 8 | 10% | 5-19 | .531 | |||
| CR2† | 22 | 4 | 18% | 8-45 | 1.94 | 0.59-6.35 | .273 | |
| Prognosis†,‡ | ||||||||
| Good prognosis† | 282 | 39 | 14% | 11-19 | .581 | |||
| Poor prognosis‡ | 61 | 10 | 16% | 9-29 | 1.21 | 0.60-2.44 | .583 | |
| MRD, all patients | ||||||||
| <1 × 10−3 | 143 | 16 | 11% | 7-18 | .855 | |||
| ≥1 × 10−3 | 20 | 2 | 10% | 3-37 | 0.87 | 0.20-3.73 | .855 | |
| Neg | 119 | 14 | 12% | 7-19 | .842 | |||
| Pos <1 × 10−3 | 24 | 2 | 8% | 2-31 | 0.66 | 0.16-2.80 | .578 | |
| Pos ≥1 × 10−3 | 20 | 2 | 10% | 3-37 | 0.82 | 0.19-3.55 | .792 | |
| MRD, only patients with ALL | ||||||||
| <1 × 10−3 | 121 | 15 | 12% | 8-20 | .743 | |||
| ≥1 × 10−3 | 11 | 1 | 9% | 1-59 | 0.72 | 0.10-5.33 | .744 | |
| Neg | 98 | 13 | 13% | 8-22 | .756 | |||
| Pos <1 × 10−3 | 23 | 2 | 9% | 2-33 | 0.61 | 0.14-2.59 | .503 | |
| Pos ≥1 × 10−3 | 11 | 1 | 9% | 1-59 | 0.66 | 0.09-4.99 | .678 | |
| MRD, only patients with AML | ||||||||
| <1 × 10−3 | 22 | 1 | 5% | 1-31 | .524 | |||
| ≥1 × 10−3 | 9 | 1 | 11% | 2-71 | 2.39 | 0.16-35.82 | .529 | |
| Neg | 21 | 1 | 5% | 1-32 | .802 | |||
| Pos ≥1 × 10−3 | 9 | 1 | 11% | 2-71 | 2.28 | 0.15-34.09 | ||
| Donor | ||||||||
| MUD | 245 | 40 | 17% | 13-22 | .085 | |||
| Haploidentical | 98 | 9 | 9% | 5-17 | 0.54 | 0.26-1.11 | .092 | |
| HLA compatibility | ||||||||
| MUD | 127 | 8 | 6% | 3-12 | <.001 | |||
| MMUD | 118 | 32 | 28% | 21-37 | 4.76 | 2.19-10-36 | <.001 | |
| Haploidentical | 98 | 9 | 9% | 5-17 | 1.46 | 0.56-3.79 | .435 | |
| Stem cell source | ||||||||
| BM | 187 | 30 | 16% | 12-23 | .267 | |||
| PBSC | 156 | 19 | 12% | 8-19 | 0.72 | 0.41-1.28 | .268 | |
| Conditioning regimen | ||||||||
| TBI-based | 204 | 31 | 16% | 11-21 | .827 | |||
| Busulfan-based | 115 | 16 | 14% | 9-22 | 0.92 | 0.50-1.67 | .774 | |
| Treosulfan-based | 21 | 2 | 10% | 3-36 | 0.64 | 0.15-2.79 | .553 | |
| Conditioning regimen, ALL patients | ||||||||
| TBI-based | 189 | 29 | 16% | 11-22 | .904 | |||
| Busulfan-based | 34 | 6 | 18% | 9-36 | 0.21 | 0.49-2.99 | .673 | |
| Treosulfan-based | 12 | 2 | 17% | 5-59 | 0.16 | 0.25-5.19 | .846 | |
| Conditioning regimen, AML patients | ||||||||
| TBI-based | 15 | 2 | 13% | 4-48 | .733 | |||
| Busulfan-based | 81 | 10 | 13% | 7-23 | 0.93 | 0.21-4.24 | ||
| Treosulfan-based | 9 | 0 | 0% | — | 0.00 | — | <.001 |
| . | Number of patients . | Events . | Cumulative incidence . | 95% CI . | P . | HR* . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|---|
| All patients | 343 | 49 | 15% | 11-19 | ||||
| Sex | ||||||||
| Male | 210 | 25 | 12% | 8-17 | .116 | |||
| Female | 133 | 24 | 18% | 13-27 | 1.57 | 0.90-2.74 | .116 | |
| Age at HSCT, y | ||||||||
| <9.5 | 172 | 15 | 9% | 6-15 | .003 | |||
| ≥9.5 | 171 | 34 | 20% | 15-27 | 2.42 | 1.32-4.45 | .004 | |
| <6 | 94 | 10 | 11% | 6-20 | .013 | |||
| 6-9.5 | 78 | 5 | 6% | 3-15 | 0.59 | 0.20-1.72 | .334 | |
| 9.5-13 | 80 | 13 | 16% | 10-27 | 1.59 | 0.69-3.65 | .274 | |
| ≥13 | 91 | 21 | 24% | 16-34 | 2.30 | 1.09-4.87 | .029 | |
| <13 | 252 | 28 | 13% | 8-16 | .006 | |||
| ≥13 | 91 | 21 | 24% | 16-34 | 2.95 | 1.25-3.84 | .006 | |
| Diagnosis | ||||||||
| ALL | 237 | 37 | 16% | 12-21 | .281 | |||
| AML | 106 | 12 | 12% | 7-20 | 0.79 | 0.37-1.34 | .280 | |
| ALL phenotype | ||||||||
| B-cell precursor | 191 | 29 | 16% | 11-22 | ||||
| T-cell precursor | 47 | 8 | 17% | 9-32 | .744 | 1.15 | 0.52-2.53 | .732 |
| Disease phase at HSCT | ||||||||
| Only ALL patients | ||||||||
| CR1† | 93 | 16 | 18% | 11-28 | .850 | |||
| CR2 S1-S2† | 85 | 11 | 13% | 8-23 | 0.72 | 0.34-1.56 | .405 | |
| CR2 S3-S4‡ | 49 | 8 | 16% | 9-31 | 0.93 | 0.40-2.17 | .865 | |
| Other CR‡ | 11 | 2 | 18% | 5-64 | 1.98 | 0.24-4.83 | .920 | |
| Only AML patients | ||||||||
| CR1† | 82 | 8 | 10% | 5-19 | .531 | |||
| CR2† | 22 | 4 | 18% | 8-45 | 1.94 | 0.59-6.35 | .273 | |
| Prognosis†,‡ | ||||||||
| Good prognosis† | 282 | 39 | 14% | 11-19 | .581 | |||
| Poor prognosis‡ | 61 | 10 | 16% | 9-29 | 1.21 | 0.60-2.44 | .583 | |
| MRD, all patients | ||||||||
| <1 × 10−3 | 143 | 16 | 11% | 7-18 | .855 | |||
| ≥1 × 10−3 | 20 | 2 | 10% | 3-37 | 0.87 | 0.20-3.73 | .855 | |
| Neg | 119 | 14 | 12% | 7-19 | .842 | |||
| Pos <1 × 10−3 | 24 | 2 | 8% | 2-31 | 0.66 | 0.16-2.80 | .578 | |
| Pos ≥1 × 10−3 | 20 | 2 | 10% | 3-37 | 0.82 | 0.19-3.55 | .792 | |
| MRD, only patients with ALL | ||||||||
| <1 × 10−3 | 121 | 15 | 12% | 8-20 | .743 | |||
| ≥1 × 10−3 | 11 | 1 | 9% | 1-59 | 0.72 | 0.10-5.33 | .744 | |
| Neg | 98 | 13 | 13% | 8-22 | .756 | |||
| Pos <1 × 10−3 | 23 | 2 | 9% | 2-33 | 0.61 | 0.14-2.59 | .503 | |
| Pos ≥1 × 10−3 | 11 | 1 | 9% | 1-59 | 0.66 | 0.09-4.99 | .678 | |
| MRD, only patients with AML | ||||||||
| <1 × 10−3 | 22 | 1 | 5% | 1-31 | .524 | |||
| ≥1 × 10−3 | 9 | 1 | 11% | 2-71 | 2.39 | 0.16-35.82 | .529 | |
| Neg | 21 | 1 | 5% | 1-32 | .802 | |||
| Pos ≥1 × 10−3 | 9 | 1 | 11% | 2-71 | 2.28 | 0.15-34.09 | ||
| Donor | ||||||||
| MUD | 245 | 40 | 17% | 13-22 | .085 | |||
| Haploidentical | 98 | 9 | 9% | 5-17 | 0.54 | 0.26-1.11 | .092 | |
| HLA compatibility | ||||||||
| MUD | 127 | 8 | 6% | 3-12 | <.001 | |||
| MMUD | 118 | 32 | 28% | 21-37 | 4.76 | 2.19-10-36 | <.001 | |
| Haploidentical | 98 | 9 | 9% | 5-17 | 1.46 | 0.56-3.79 | .435 | |
| Stem cell source | ||||||||
| BM | 187 | 30 | 16% | 12-23 | .267 | |||
| PBSC | 156 | 19 | 12% | 8-19 | 0.72 | 0.41-1.28 | .268 | |
| Conditioning regimen | ||||||||
| TBI-based | 204 | 31 | 16% | 11-21 | .827 | |||
| Busulfan-based | 115 | 16 | 14% | 9-22 | 0.92 | 0.50-1.67 | .774 | |
| Treosulfan-based | 21 | 2 | 10% | 3-36 | 0.64 | 0.15-2.79 | .553 | |
| Conditioning regimen, ALL patients | ||||||||
| TBI-based | 189 | 29 | 16% | 11-22 | .904 | |||
| Busulfan-based | 34 | 6 | 18% | 9-36 | 0.21 | 0.49-2.99 | .673 | |
| Treosulfan-based | 12 | 2 | 17% | 5-59 | 0.16 | 0.25-5.19 | .846 | |
| Conditioning regimen, AML patients | ||||||||
| TBI-based | 15 | 2 | 13% | 4-48 | .733 | |||
| Busulfan-based | 81 | 10 | 13% | 7-23 | 0.93 | 0.21-4.24 | ||
| Treosulfan-based | 9 | 0 | 0% | — | 0.00 | — | <.001 |
Subgroups including fewer than 5 patients were not shown in the table.
The Cox regression model was used to estimate the HR, and the first subgroup of patients of each independent variable was used as reference value.
The good prognosis group included patients affected by ALL in first CR, ALL in second CR after a relapse belonging to the S1-S2 risk groups and patients with AML in first CR.
The poor prognosis group included patients affected by ALL in second CR after a relapse belonging to the S3-S4 risk groups, ALL in third or subsequent CR, and patients with AML in second CR.
Multivariable analysis of factors affecting NRM, cumulative incidence of relapse and LFS
| Variable . | Risk ratio . | 95% CI . | P . |
|---|---|---|---|
| Nonrelapse mortality | |||
| Age at HSCT | |||
| ≥13 y vs <13 y | 1.88 | 1.05-3.35 | .033 |
| Donor | |||
| MMUD vs MUD | 4.35 | 1.97-9.60 | <.001 |
| Haploidentical vs MUD | 1.37 | 0.52-3.56 | .523 |
| Relapse | |||
| Diagnosis | |||
| AML vs ALL | 1.20 | 0.52-3.46 | .737 |
| Prognosis | |||
| Poor vs good | 3.43 | 1.69-6.94 | .001 |
| MRD | |||
| ≥1 × 10−3 vs <1 × 10−3 | 2.97 | 1.32-6.67 | .008 |
| Conditioning regimen | |||
| Busulfan vs TBI | 1.51 | 0.51-4.45 | .452 |
| Treosulfan vs TBI | 1.46 | 0.64-3.56 | .370 |
| Donor | |||
| MMUD vs MUD | 0.52 | 0.25-1.10 | .088 |
| Haploidentical vs MUD | 0.71 | 0.33-1.54 | .383 |
| Leukemia-free-survival | |||
| Sex | |||
| Female vs male | 1.63 | 0.97-2.75 | .065 |
| Age at HSCT | |||
| ≥13 y vs <13 y | 0.94 | 0.52-1.72 | .842 |
| Diagnosis | |||
| AML vs ALL | 0.81 | 0.33-1.98 | .641 |
| Prognosis | |||
| Poor vs good | 3.00 | 169-5.31 | <.001 |
| MRD level | |||
| ≥1 × 10−3 vs <1 × 10−3 | 2.90 | 1.47-5.71 | .002 |
| Conditioning regimen | |||
| Busulfan vs TBI | 1.22 | 0.52-2.86 | .648 |
| Treosulfan vs TBI | 1.45 | 0.64-3.27 | .372 |
| Donor | |||
| MMUD vs MUD | 0.93 | 0.51-1.69 | .800 |
| Haploidentical vs MUD | 0.69 | 0.36-1.32 | .258 |
| Variable . | Risk ratio . | 95% CI . | P . |
|---|---|---|---|
| Nonrelapse mortality | |||
| Age at HSCT | |||
| ≥13 y vs <13 y | 1.88 | 1.05-3.35 | .033 |
| Donor | |||
| MMUD vs MUD | 4.35 | 1.97-9.60 | <.001 |
| Haploidentical vs MUD | 1.37 | 0.52-3.56 | .523 |
| Relapse | |||
| Diagnosis | |||
| AML vs ALL | 1.20 | 0.52-3.46 | .737 |
| Prognosis | |||
| Poor vs good | 3.43 | 1.69-6.94 | .001 |
| MRD | |||
| ≥1 × 10−3 vs <1 × 10−3 | 2.97 | 1.32-6.67 | .008 |
| Conditioning regimen | |||
| Busulfan vs TBI | 1.51 | 0.51-4.45 | .452 |
| Treosulfan vs TBI | 1.46 | 0.64-3.56 | .370 |
| Donor | |||
| MMUD vs MUD | 0.52 | 0.25-1.10 | .088 |
| Haploidentical vs MUD | 0.71 | 0.33-1.54 | .383 |
| Leukemia-free-survival | |||
| Sex | |||
| Female vs male | 1.63 | 0.97-2.75 | .065 |
| Age at HSCT | |||
| ≥13 y vs <13 y | 0.94 | 0.52-1.72 | .842 |
| Diagnosis | |||
| AML vs ALL | 0.81 | 0.33-1.98 | .641 |
| Prognosis | |||
| Poor vs good | 3.00 | 169-5.31 | <.001 |
| MRD level | |||
| ≥1 × 10−3 vs <1 × 10−3 | 2.90 | 1.47-5.71 | .002 |
| Conditioning regimen | |||
| Busulfan vs TBI | 1.22 | 0.52-2.86 | .648 |
| Treosulfan vs TBI | 1.45 | 0.64-3.27 | .372 |
| Donor | |||
| MMUD vs MUD | 0.93 | 0.51-1.69 | .800 |
| Haploidentical vs MUD | 0.69 | 0.36-1.32 | .258 |
Subgroups including fewer than 5 patients were not shown in the table.
Seventy-five (24%) children relapsed at a median time of 190 days (range, 40-1603 days) after the allograft (Figure 3A). No statistically significant difference for the cumulative incidence of disease recurrence was observed among MUD-HSCT, MMUD-HSCT, and αβhaplo-HSCT recipients (26% vs 17% vs 29%, respectively; see supplemental Table 4 for more details). A poor disease risk (HR, 3.43; 95% CI, 1.69-6.94; P = .001) and a high pre-HSCT MRD level (HR, 2.97; 95% CI, 1.32-6.67; P = .008) were associated with increased risk for relapse in the multivariable analysis, as detailed in Table 3.
Survival, LFS, and GFRS
With a median follow-up of 3.3 years (range, 1.5-7 years for surviving patients), the 5-year probability of OS for patients given either UD-HSCT or αβhaplo-HSCT is 64% and 68%, respectively (P = N.S.; supplemental Table 1). The 5-year probability of LFS is 65%, 55%, and 62% in MUD, MMUD, and αβhaplo-HSCT, respectively (P = N.S.; Figure 3C). In univariable analysis, an advanced disease phase at HSCT, MRD level higher than 1 × 10−3 30 days before HSCT and a Treosulfan-based conditioning regimen in ALL were associate with poorer outcome (P < .0001, P = .003, and P = .004, respectively; see Table 4 for details). In multivariable analysis, the disease risk stratification (HR, 3.43; 95% CI, 1.69-6.94l; P = .001) and MRD level before HSCT remained significant (HR, 2.97; 95% CI, 1.32-6.67; P = .008, Table 3). In the αβhaplo-HSCT recipients, the 5-year LFS of patients transplanted from either an NK-alloreactive or nonalloreactive donor was superimposable (60% vs 64%, respectively; P = N.S.). The 5-year chronic GRFS in the UD-HSCT and αβhaplo-HSCT groups was 48% and 59%, respectively (P = .03; supplemental Table 1). Notably, chronic GRFS of MUD-HSCT and αβhaplo-HSCT recipients was superimposable (61% and 58%, respectively), whereas the choice of MMUD-HSCT had a detrimental effect on this composite end point (34%; P < .001; Figure 3D).
Univariable analysis of LFS in the cohort of patients analyzed
| . | Number of patients . | Events . | Probability . | 95% CI . | P . | HR* . | (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| All patients | 343 | 124 | 62% | 56-67 | ||||
| Sex | ||||||||
| Male | 210 | 66 | 66% | 58-73 | .015 | |||
| Female | 133 | 58 | 55% | 47-64 | 1.54 | 1.07-2.20 | .016 | |
| Age at HSCT, y | ||||||||
| <9.5 | 172 | 57 | 65% | 58-73 | .218 | |||
| ≥9.5 | 171 | 67 | 58% | 50-66 | 1.25 | 0.88-1.78 | .218 | |
| <6 | 94 | 33 | 64% | 54-74 | .165 | |||
| 6-9.5 | 78 | 24 | 66% | 54-78 | 0.82 | 0.48-1.39 | .460 | |
| 9.5-13 | 80 | 26 | 65% | 53-77 | 0.91 | 0.54-1.51 | .705 | |
| ≥13 | 91 | 41 | 52% | 41-63 | 1.37 | 0.87-2.17 | .178 | |
| <13 | 252 | 83 | 65% | 59-72 | .032 | |||
| ≥13 | 91 | 41 | 52% | 41-63 | 1.50 | 1.03-2.18 | .033 | |
| Diagnosis | ||||||||
| ALL | 237 | 93 | 59% | 52-66 | .064 | |||
| AML | 106 | 31 | 67% | 57-78 | 0.68 | 0.45-1.03 | .066 | |
| ALL phenotype | ||||||||
| B-cell precursor | 191 | 74 | 59% | 50-66 | .713 | |||
| T-cell precursor | 47 | 19 | 59% | 45-73 | 0.91 | 0.55-1.50 | .713 | |
| Disease phase at HSCT | ||||||||
| Only ALL patients | ||||||||
| CR1† | 93 | 31 | 66.7% | 56-76 | .0005 | |||
| CR2 S1-S2† | 85 | 26 | 69.4% | 58-79 | 0.89 | 0.53-1.49 | .651 | |
| CR2 S3-S4‡ | 49 | 29 | 40.8% | 24-53 | 2.11 | 1.27-3.51 | .004 | |
| Other CR‡ | 11 | 7 | 36.4% | 8-64 | 2.74 | 1.21-6.24 | .016 | |
| Only AML patients | ||||||||
| CR1† | 82 | 23 | 72% | 56-80 | .084 | |||
| CR2† | 22 | 7 | 68.2% | 48-88 | 1.25 | 0.54-2.91 | .607 | |
| Prognosis†,‡ | ||||||||
| Good prognosis† | 282 | 87 | 67% | 62-73 | .0001 | |||
| Poor prognosis‡ | 61 | 37 | 37% | 23-50 | 2.54 | 1.73-3.74 | .0001 | |
| MRD, all patients | ||||||||
| <1 × 10−3 | 143 | 52 | 63% | 54-71 | ||||
| ≥1 × 10−3 | 20 | 13 | 20% | 0-49 | .003 | 2.48 | 1.35-4.56 | .004 |
| Neg | 119 | 39 | 66% | 58-75 | ||||
| Pos <1 × 10−3 | 24 | 13 | 42% | 20-63 | 1.68 | 0.90-3.16 | .105 | |
| Pos ≥1 × 10−3 | 20 | 16 | 20% | 0-49 | .003 | 2.76 | 1.47-5.19 | .002 |
| MRD, only ALL patients | ||||||||
| <1 × 10−3 | 121 | 46 | 61% | 52-70 | ||||
| ≥1 × 10−3 | 11 | 7 | 36% | 8-65 | .046 | 2.20 | 0.99-4.88 | .052 |
| Neg | 98 | 33 | 66% | 57-75 | ||||
| Pos <1 × 10−3 | 23 | 13 | 39% | 17-61 | 1.70 | 0.89-3.23 | .110 | |
| Pos ≥1 × 10−3 | 11 | 7 | 36% | 8-65 | .040 | 2.49 | 1.10-5.64 | .028 |
| MRD, only AML patients | ||||||||
| <1 × 10−3 | 22 | 6 | 72% | 52-91 | ||||
| ≥1 × 10−3 | 9 | 6 | 0% | — | .010 | 4.14 | 1.28-13.38 | .017 |
| Neg | 21 | 6 | 70% | 50-90 | ||||
| Pos ≥1 × 10−3 | 9 | 9 | 0% | — | .033 | 3.92 | 1.22-12.65 | .022 |
| Donor | ||||||||
| MUD | 245 | 90 | 63.3% | 65-68 | .650 | |||
| Haploidentical | 98 | 34 | 65.3% | 51-73 | 1095 | 0.74-1.63 | .650 | |
| HLA compatibility | ||||||||
| MUD | 127 | 40 | 67% | 59-76 | .150 | |||
| MMUD | 118 | 50 | 55% | 45-65 | 1.48 | 0.98-2.25 | .063 | |
| Haploidentical | 98 | 34 | 62% | 51-73 | 1.11 | 0.71-1.76 | .642 | |
| Stem cell source | ||||||||
| BM | 187 | 68 | 64% | 55-69 | .731 | |||
| PBSC | 156 | 56 | 64% | 53-70 | 1.064 | 0.75-1.52 | .731 | |
| Conditioning regimen | ||||||||
| TBI-based | 204 | 73 | 64% | 54-69 | .673 | |||
| Busulfan-based | 115 | 40 | 65% | 53-72 | 0.95 | 0.64-1.39 | .783 | |
| Treosulfan-based | 21 | 10 | 52% | 28-72 | 1.45 | 0.75-2.81 | .270 | |
| Conditioning regimen, ALL patients | ||||||||
| TBI-based | 189 | 69 | 61% | 53-69 | .004 | |||
| Busulfan-based | 34 | 14 | 59% | 42-75 | 1.20 | 0.68-2.13 | .532 | |
| Treosulfan-based | 12 | 10 | 17% | 0-38 | 3.11 | 1.60-6.06 | .001 | |
| Conditioning regimen, AML patients | ||||||||
| TBI-based | 15 | 4 | 73% | 51-96 | .155 | |||
| Busulfan-based | 81 | 26 | 65% | 53-76 | 1.14 | 0.40-3.27 | .805 | |
| Treosulfan-based | 9 | 0 | 100% | — | 0.00 | — | <.001 |
| . | Number of patients . | Events . | Probability . | 95% CI . | P . | HR* . | (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| All patients | 343 | 124 | 62% | 56-67 | ||||
| Sex | ||||||||
| Male | 210 | 66 | 66% | 58-73 | .015 | |||
| Female | 133 | 58 | 55% | 47-64 | 1.54 | 1.07-2.20 | .016 | |
| Age at HSCT, y | ||||||||
| <9.5 | 172 | 57 | 65% | 58-73 | .218 | |||
| ≥9.5 | 171 | 67 | 58% | 50-66 | 1.25 | 0.88-1.78 | .218 | |
| <6 | 94 | 33 | 64% | 54-74 | .165 | |||
| 6-9.5 | 78 | 24 | 66% | 54-78 | 0.82 | 0.48-1.39 | .460 | |
| 9.5-13 | 80 | 26 | 65% | 53-77 | 0.91 | 0.54-1.51 | .705 | |
| ≥13 | 91 | 41 | 52% | 41-63 | 1.37 | 0.87-2.17 | .178 | |
| <13 | 252 | 83 | 65% | 59-72 | .032 | |||
| ≥13 | 91 | 41 | 52% | 41-63 | 1.50 | 1.03-2.18 | .033 | |
| Diagnosis | ||||||||
| ALL | 237 | 93 | 59% | 52-66 | .064 | |||
| AML | 106 | 31 | 67% | 57-78 | 0.68 | 0.45-1.03 | .066 | |
| ALL phenotype | ||||||||
| B-cell precursor | 191 | 74 | 59% | 50-66 | .713 | |||
| T-cell precursor | 47 | 19 | 59% | 45-73 | 0.91 | 0.55-1.50 | .713 | |
| Disease phase at HSCT | ||||||||
| Only ALL patients | ||||||||
| CR1† | 93 | 31 | 66.7% | 56-76 | .0005 | |||
| CR2 S1-S2† | 85 | 26 | 69.4% | 58-79 | 0.89 | 0.53-1.49 | .651 | |
| CR2 S3-S4‡ | 49 | 29 | 40.8% | 24-53 | 2.11 | 1.27-3.51 | .004 | |
| Other CR‡ | 11 | 7 | 36.4% | 8-64 | 2.74 | 1.21-6.24 | .016 | |
| Only AML patients | ||||||||
| CR1† | 82 | 23 | 72% | 56-80 | .084 | |||
| CR2† | 22 | 7 | 68.2% | 48-88 | 1.25 | 0.54-2.91 | .607 | |
| Prognosis†,‡ | ||||||||
| Good prognosis† | 282 | 87 | 67% | 62-73 | .0001 | |||
| Poor prognosis‡ | 61 | 37 | 37% | 23-50 | 2.54 | 1.73-3.74 | .0001 | |
| MRD, all patients | ||||||||
| <1 × 10−3 | 143 | 52 | 63% | 54-71 | ||||
| ≥1 × 10−3 | 20 | 13 | 20% | 0-49 | .003 | 2.48 | 1.35-4.56 | .004 |
| Neg | 119 | 39 | 66% | 58-75 | ||||
| Pos <1 × 10−3 | 24 | 13 | 42% | 20-63 | 1.68 | 0.90-3.16 | .105 | |
| Pos ≥1 × 10−3 | 20 | 16 | 20% | 0-49 | .003 | 2.76 | 1.47-5.19 | .002 |
| MRD, only ALL patients | ||||||||
| <1 × 10−3 | 121 | 46 | 61% | 52-70 | ||||
| ≥1 × 10−3 | 11 | 7 | 36% | 8-65 | .046 | 2.20 | 0.99-4.88 | .052 |
| Neg | 98 | 33 | 66% | 57-75 | ||||
| Pos <1 × 10−3 | 23 | 13 | 39% | 17-61 | 1.70 | 0.89-3.23 | .110 | |
| Pos ≥1 × 10−3 | 11 | 7 | 36% | 8-65 | .040 | 2.49 | 1.10-5.64 | .028 |
| MRD, only AML patients | ||||||||
| <1 × 10−3 | 22 | 6 | 72% | 52-91 | ||||
| ≥1 × 10−3 | 9 | 6 | 0% | — | .010 | 4.14 | 1.28-13.38 | .017 |
| Neg | 21 | 6 | 70% | 50-90 | ||||
| Pos ≥1 × 10−3 | 9 | 9 | 0% | — | .033 | 3.92 | 1.22-12.65 | .022 |
| Donor | ||||||||
| MUD | 245 | 90 | 63.3% | 65-68 | .650 | |||
| Haploidentical | 98 | 34 | 65.3% | 51-73 | 1095 | 0.74-1.63 | .650 | |
| HLA compatibility | ||||||||
| MUD | 127 | 40 | 67% | 59-76 | .150 | |||
| MMUD | 118 | 50 | 55% | 45-65 | 1.48 | 0.98-2.25 | .063 | |
| Haploidentical | 98 | 34 | 62% | 51-73 | 1.11 | 0.71-1.76 | .642 | |
| Stem cell source | ||||||||
| BM | 187 | 68 | 64% | 55-69 | .731 | |||
| PBSC | 156 | 56 | 64% | 53-70 | 1.064 | 0.75-1.52 | .731 | |
| Conditioning regimen | ||||||||
| TBI-based | 204 | 73 | 64% | 54-69 | .673 | |||
| Busulfan-based | 115 | 40 | 65% | 53-72 | 0.95 | 0.64-1.39 | .783 | |
| Treosulfan-based | 21 | 10 | 52% | 28-72 | 1.45 | 0.75-2.81 | .270 | |
| Conditioning regimen, ALL patients | ||||||||
| TBI-based | 189 | 69 | 61% | 53-69 | .004 | |||
| Busulfan-based | 34 | 14 | 59% | 42-75 | 1.20 | 0.68-2.13 | .532 | |
| Treosulfan-based | 12 | 10 | 17% | 0-38 | 3.11 | 1.60-6.06 | .001 | |
| Conditioning regimen, AML patients | ||||||||
| TBI-based | 15 | 4 | 73% | 51-96 | .155 | |||
| Busulfan-based | 81 | 26 | 65% | 53-76 | 1.14 | 0.40-3.27 | .805 | |
| Treosulfan-based | 9 | 0 | 100% | — | 0.00 | — | <.001 |
Subgroups including fewer than 5 patients were not shown in the table.
The Cox regression model was used to estimate the HR, and the first subgroup of patients of each independent variable was used as reference value.
The good prognosis group included patients affected by ALL in first CR, ALL in second CR after a relapse belonging to the S1-S2 risk groups and patients with AML in first CR.
The poor-prognosis group included patients affected by ALL in second CR after a relapse belonging to the S3-S4 risk groups, ALL in third or subsequent CR, and patients with AML in second CR.
Discussion
This study represents the first multicenter, retrospective comparative analysis on AL children receiving either UD-HSCT or αβhaplo-HSCT. Indeed, no comparative data are available for pediatric patients, although, in adults, some studies have compared the outcome of UD-HSCT with that of unmanipulated HLA-haploidentical HSCT.42-44
Recently, several single-center reports demonstrated the safety and efficacy of the αβ T-cell/CD19+ B-cell depletion as a graft-engineering approach in children receiving HLA-haploidentical HSCT.23-25 The present analysis definitely establish on a large/multisite scale that αβhaplo-HSCT represents an equally effective option to MUD-HSCT for children with AL lacking a sibling donor.
As reported in Table 1, the majority of patients enrolled in our study had ALL (59%). Remarkably, αβhaplo-HSCT recipients were transplanted in a more advanced disease phase and more frequently received a TBI-based conditioning regimen (74%) as compared with the MUD (50%) and MMUD-HSCT groups (58%; Table 1). In fact, αβhaplo-HSCT was offered to children who lacked either an HLA-identical sibling or a MUD, or in case of a rapidly progressive or unstable disease not allowing the time to identify a suitable MUD. Remarkably, the incidence of graft failure in the αβhaplo-HSCT did not differ from that of patients given UD-HSCT. Consistent with what we reported in a recent single-center trial,25 none of the αβhaplo-HSCT recipients experienced either grade III to IV or visceral acute GVHD (Figure 2B). The advantage of αβhaplo-HSCT is even more evident if we compare the cumulative incidence of the more severe form of acute GVHD in recipients of αβhaplo-HSCT and that in recipients of MMUD-HSCT (Figure 2B). Moreover, in the αβhaplo-HSCT cohort, all cases but 1 of chronic GVHD were of limited severity (Figure 2D). The low risk of developing severe acute GVHD likely contributed to a lower risk for NRM in MUD and αβhaplo-HSCT recipients in comparison with MMUD-HSCT (P < .001; Figure 3B). Notably, the median number of residual αβ T cells in the haploidentical grafts was very low, being 0.04 × 106/kg body weight. In light of the direct correlation between αβ T cells and GVHD, we believe that an optimal graft composition is crucial for the outcome of the αβhaplo-HSCT. Thus, we strongly recommend maintaining a threshold of residual αβ T cells lower than 1 × 105/kg recipient body weight. In the αβhaplo-HSCT group, the ex vivo CD19+ depletion coupled with the in vivo CD20+ depletion obtained through the pretransplant use of rituximab could explain the total absence of EBV-related PTLD, a fearsome complication typical of immunocompromised patients. Moreover, we can speculate that the use of rituximab on day −1 helped prevent the occurrence not only of EBV-PTLD but also that of GVHD.45
In the last 20 years, multiple strategies of ex vivo and in vivo T-cell depletion techniques have been reported to be effective in HLA-haploidentical HSCT.46,47 In addition to the pioneering studies using CD34+ positive selection,13,14 some centers reported data on combined CD3+ and CD19+ negative depletion.48-50 Both these approaches resulted in the loss of some cell subsets playing a crucial role in infection defense and leukemia recurrence prevention, translating into a higher risk for NRM and disease relapse. After 2010, several centers, mostly European, started to use the αβ T-cell/CD19+ B-cell depletion approach.15 The goal of this new method of graft manipulation is to leave in the graft not only HSCs but also cells pertaining to innate immunity, which might facilitate engraftment and reduce risk for both infections and leukemia recurrence.16,17 In fact, although T cells carrying the αβ T-cell receptor (TCR) are responsible for GVHD,51,52 γδ T cells have no alloreactive capacity.53 However, they can contribute to an important anti-infectious54 and antileukemia effect.21,55-57 Thanks to this technique, patients can also immediately benefit from donor NK cells contained in this type of graft that can fully exert their activity, filling the 6- to 8-week gap documented in previous studies before mature KIR+ NK cells differentiating from CD34+ cells are consistently detected.19,32 Altogether, the infusion with the graft of these different lymphoid cell subsets could explain our finding that NRM and LFS were superimposable when results of αβhaplo HSCT and MUD-HSCT are compared. Notably, the incidence of fatal events resulting from severe infections was equal in the MUD and αβhaplo-HSCT group, highlighting the role of both NK and γδ cells in protecting the host in the very early phase after the procedure in the αβhaplo-HSCT group. Conversely, in the MMUD-HSCT cohort, most deaths (33%) were a result of infections, which were mainly bacterial (supplemental Tables 2 and 3). In addition, the infusion with the graft of a large number of such effector cells, along with a megadose of HSCs and with the use of a fully myeloablative conditioning regimen, might explain the remarkably low incidence of graft failure (2%) observed in our αβhaplo-HSCT group. Moreover, the graft composition granted a faster engraftment of neutrophils and platelets in the αβhaplo-HSCT group. This last finding can, at least partly, be responsible of the significantly lower incidence of bacterial infections in the αβhaplo-HSCT (8%) group in comparison with the MUD (17%) and the MMUD-HSCT (34%) groups (P < .001; supplemental Table 3).
Ultimately, the absence of pharmacological post-HSCT GVHD prophylaxis in our haploidentical cohort and the use of a fully myeloablative conditioning regimen could explain the lower incidence of relapse in comparison with previously published single-center reports. Indeed, with a median follow-up of 1.6 years, Lang et al showed a 41.4% cumulative incidence of leukemia recurrence in a cohort of 41 pediatric patients transplanted after a reduced-intensity, TBI-free conditioning regimen.23 Maschan et al analyzed the outcome of children with high-risk AML receiving αβ T-cell-depleted HSCT either from an UD or a haploidentical donor.24 The majority of patients received different combination of post-HSCT pharmacological prophylaxis. The 2-year cumulative incidence of relapse in their haplo cohort was 40%. Although even in our study the main cause of treatment failure is relapse after αβhaplo-HSCT (cumulative incidence, 24%; Figure 2A; supplemental Table 4 for more details), we can speculate that NK cells and other innate lymphoid cells not exposed to immunosuppressive treatment could display a better antileukemia effect leading to superior LFS.
In contrast to what was observed with the CD34+ positive selection approach, but consistent with our single-center experience data recently published,25 in our αβhaplo-HSCT cohort, the use of an NK alloreactive donor did not affect the leukemia-free survival. Likely, we can speculate that infusion of γδ T cells with the graft can obscure the role played by NK alloreactivity in a contest of extensive and prolonged T-cell depletion.
In multivariable analysis, belonging to a good-prognosis risk group and an MRD level lower than 1 × 10−3 before HSCT had a positive effect on LFS (HR, 3.43 [95% CI, 1.69-6.94l; P = .001]; HR, 2.97 [95% CI, 1.32-6.67; P = .008], respectively). These findings confirm the importance of obtaining the best possible leukemia control already reported from different groups, regardless of donor type or graft-manipulation strategy.58,59 The recent implementation of next-generation sequencing approaches for evaluating MRD may further affect the success of HSCT, allowing us to implement strategies aimed at preventing recurrence of leukemia.60,61 In this multicenter analysis, contrary to what was observed in the single-center experience we previously reported,25 the type of conditioning regimen employed (TBI-based or chemotherapy-based) did not influence LFS in multivariable analysis (Table 4). As chemotherapy, we mainly employed a Busulfan-based conditioning regimen. Only a very small proportion of patients received Treosulfan as a part of the preparative regimen (6%, 5%, and 7% in the MUD, MMUD, and αβhaplo-HSCT groups, respectively). Although the relapse incidence of patients with ALL receiving Treosulfan is higher as compared with the other regimens (supplemental Table 4), in view of the low number of patients treated according to this chemotherapy, no definitive conclusion can be drawn.
In parallel to different graft-engineering approaches, in vivo strategies of T-cell depletion based on the posttransplant infusion of Cyclophosphamide have been developed in the setting of haplo-HSCT.62 Although largely used in adults, little is known on the use of this method in children.63,64 The few studies so far available reported a risk for leukemia recurrence higher than the one observed in this multicenter analysis.64
In conclusion, our study, the first in a multicenter setting, confirms that αβ T-cell– and B-cell–depleted HLA-haploidentical HSCT is currently a valuable option for children with AL lacking a sibling donor. First, this approach is associated with a cumulative incidence of NRM and disease recurrence comparable to that of children transplanted from a MUD. Second, αβ T-cell and B-cell depletion abrogates the risk of developing severe acute and chronic GVHD and is also associated with faster neutrophil and platelet recovery than UD-HSCT. Finally, when compared with MMUD-HSCT, αβhaplo-HSCT is clearly superior, showing a significantly lower cumulative incidence of NRM and a better chronic GFRS. As noted previously, an alternative treatment using posttransplant Cyclophosphamide as an in vivo T-cell depletion approach have shown some encouraging results,62,65 although published work, to date, has focused primarily on adults. Prospective trials comparing this approach with αβ T-cell and B-cell depletion are needed in children to establish which alternative represents the optimal treatment under which conditions. In the meantime, data from the present study support the argument that αβhaplo-HSCT should be considered a transplant option of equal efficacy vs MUD-HSCT and better than MMUD-HSCT for AL children in need of an allograft and lacking a fully matched related donor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from AIRC (Special Grant ”5xmille“-9962 to F.L.; investigator grant IG 17200 to F.L.; ”My first AIRC“ grant 15925 to A. Bertaina), Ministero della Salute (RF-2010-2316606 to F.L.; Ricerca Corrente to F.L.), Regione Lazio (Grant FILAS to F.L.), and Ministero dell’Istruzione, Università e Ricerca (Grant Progetto di Rilevante Interesse Nazionale, PRIN 2010, to F.L.).
Authorship
Contribution: A. Bertaina, M.Z., and F.L. provided conception and design; A. Bertaina, M.Z., M.A., F.S., C.P., E.L., A.P., W.B., M.T., C.F., S.C., F.D.B., M.R., S.B., G.C., M.R., A. Balduzzi, F.F., D.P., and F.L. provided transplantation of patients and collection of data; N.S. and A.M.G. provided unrelated donor search and selection; B.B. provided minimal residual disease monitoring; V.B. provided immune monitoring; A. Bertaina, M.Z., D.P., and F.L. provided analysis and interpretation of data (eg, statistical analysis, computational analysis); and all authors provided writing, review and/or revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alice Bertaina, Division of Stem Cell Transplant and Regenerative Medicine, Department of Pediatrics, School of Medicine, Stanford University, 1000 Welch Rd, Stanford, CA, 94305; e-mail: aliceb1@stanford.edu.
REFERENCES
Author notes
A. Bertaina and M.Z. are joint first authors.
D.P. and F.L. are joint senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal