TO THE EDITOR:
Borrelia burgdorferi sensu lato complex (Bb sl) is a group of Borrelia species that is responsible for Lyme disease transmitted to humans by Ixodes tick bites. Lyme borreliosis is an emerging zoonosis and the most important vector-borne disease of the northern hemisphere, with 30 000 to 300 000 cases reported each year in the United States1 and 65 000 cases per year in Europe.2 The association between chronic bacterial infections and lymphomas has been suggested for a long time; however, with the exception of a few pathologies (ie, gastric B-cell lymphomas and Helicobacter pylori infection3 ), clear demonstrations of causative links are missing. Although various Borrelia strains have been associated with primary cutaneous lymphomas, the results have been almost exclusively reported as case studies or small retrospective series of cutaneous lymphomas4-7 and remain controversial.8,9 Bb sl DNA has been detected in 10% to 42% of patients with cutaneous mucosa-associated lymphoid tissue B-cell lymphomas, in 15% to 26% of cutaneous follicular and diffuse large B-cell lymphomas,5,6 and in 18% of mycosis fungoides (MFs) diagnosed in some European countries.7,10 Taken together, there is insufficient evidence to show a definitive association between Bb sl and lymphomas. Therefore, the development of animal models to show the direct link between Borrelia-driven infection and lymphomagenesis is required.
To explore the role of Bb sl in T-cell lymphomagenesis, we used p53−/− mice that develop, in addition to the well-known immature thymic T-cell lymphomas,11,12 spontaneous peripheral T-cell lymphomas (PTCLs) originating from natural killer T (NKT) cells.13 All animal studies and procedures were performed in accordance with European Union guidelines and were approved by the Animal Ethics Evaluation Committee.
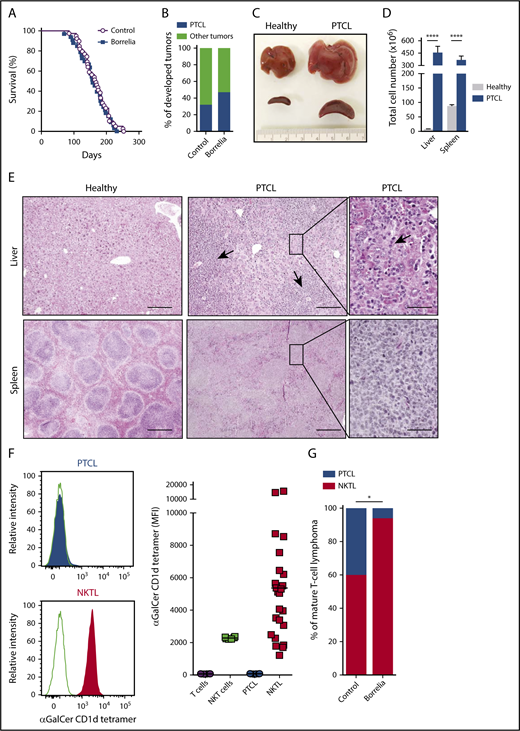
p53−/− mice were injected with live Borrelia afzelii IBS39 (n = 34) or with control medium (Barbour–Stoenner–Kelly medium; n = 39) intradermally to mimic tick bites. Of the 34 mice injected with B afzelii, 29 tested positive for anti-Borrelia immunoglobulin G (IgG) 15 and 30 days postinjection, confirming the infection. The median survival of 158 days was not significantly different from uninfected p53−/− mice (median survival = 168 days) (Figure 1A). Among Borrelia-infected mice, almost 50% developed CD19−, CD3+, Thy1+ PTCL compared with only 32% in the uninfected group (Figure 1B; supplemental Figure 1, available on the Blood Web site). Macroscopically, these PTCLs were characterized by hepatomegaly and splenomegaly (Figure 1C), with a 58-fold and fourfold increase in lymphocytes in liver and spleen, respectively, compared with healthy mice (Figure 1D). The architecture of spleen and liver from PTCL mice was nodular and diffuse, with massive cell infiltration leading to effacement of the normal structure (Figure 1E). The PTCL infiltrate in the liver was massively perivascular but also intrasinusoidal, the later being where normal NKT cells are preferentially found in healthy liver (Figure 1E). Because Borrelia-expressed glycolipids enable direct NKT cell activation and expansion,14 we studied the NKT cell origin of these PTCLs using α-Galactosylceramide (αGalCer)-loaded CD1d tetramer. A set of PTCLs was characterized by positive CD1d tetramer staining, defining NKT lymphomas (NKTLs), whereas another set of PTCLs was negative, indicating conventional PTCL (Figure 1F; supplemental Figure 1; supplemental Table 1). As shown in Figure 1G, Borrelia infection significantly increased the incidence of NKTL (94% in the Borrelia-infected group vs 61% in the uninfected group). The NKT cell origin of these PTCLs was further validated by expression of the invariant Vα14-Jα18 rearrangement of the T-cell receptor α (TCRα) chain and by the rearrangement of the TCRβ chain with only Vβ7, Vβ8.2, and Vβ8.3 rearrangements (supplemental Table 1). In the Borrelia-infected group, NKTLs were clonal, with a clone-productive frequency of the TCRβ chain rearrangement between 84.9% and 99.8% (supplemental Table 1).
Borrelia infection increases the frequency of PTCL arising from NKT cells. (A) Survival curves of p53−/− mice infected with 5 × 104 spirochetes (n = 29) or control medium (n = 39). (B) Bar graph shows the tumor spectra developed in infected and control p53−/− mice. Other tumors include solid tumors and thymic lymphomas. (C) PTCLs are characterized by hepatosplenomegaly. Macroscopic images of livers and spleens from PTCL-bearing and healthy p53−/− mice. (D) PTCLs show a significant increase in cells in liver and spleen. Data are mean ± SD. ****P < .0001, Mann-Whitney U test. (E) Hematoxylin phloxine saffron (HPS) staining of paraffin-embedded organ sections from healthy and PTCL-bearing p53−/− mice. Arrows in middle panel indicate perivascular infiltrates of lymphoma cells. Arrow in the right panel denoted intrasinusoidal infiltrate of lymphoma cells. Scale bars, 200 µm (left and middle panels), 50 µm (right panels). (F) p53−/− mice develop 2 types of PTCL. Flow cytometry analysis of PTCLs (gated on the CD3+ Thy1.2+ population) stained with αGalCer-loaded CD1d tetramers. Line graphs are representative of 2 PTCL types: CD1d tetramer–negative PTCL (gray shading; PTCL) and CD1d tetramer–positive PTCL (red shading; NKTL) (left panels). CD1d tetramer staining of all PTCLs compared with normal T and NKT cells (right panel). (G) Borrelia infection increases the frequency of NKTL. PTCL and NKTL frequency among all PTCLs in control and Borrelia-infected p53−/− mice with significantly different PTCL spectra. *P < .02, χ2 test.
Borrelia infection increases the frequency of PTCL arising from NKT cells. (A) Survival curves of p53−/− mice infected with 5 × 104 spirochetes (n = 29) or control medium (n = 39). (B) Bar graph shows the tumor spectra developed in infected and control p53−/− mice. Other tumors include solid tumors and thymic lymphomas. (C) PTCLs are characterized by hepatosplenomegaly. Macroscopic images of livers and spleens from PTCL-bearing and healthy p53−/− mice. (D) PTCLs show a significant increase in cells in liver and spleen. Data are mean ± SD. ****P < .0001, Mann-Whitney U test. (E) Hematoxylin phloxine saffron (HPS) staining of paraffin-embedded organ sections from healthy and PTCL-bearing p53−/− mice. Arrows in middle panel indicate perivascular infiltrates of lymphoma cells. Arrow in the right panel denoted intrasinusoidal infiltrate of lymphoma cells. Scale bars, 200 µm (left and middle panels), 50 µm (right panels). (F) p53−/− mice develop 2 types of PTCL. Flow cytometry analysis of PTCLs (gated on the CD3+ Thy1.2+ population) stained with αGalCer-loaded CD1d tetramers. Line graphs are representative of 2 PTCL types: CD1d tetramer–negative PTCL (gray shading; PTCL) and CD1d tetramer–positive PTCL (red shading; NKTL) (left panels). CD1d tetramer staining of all PTCLs compared with normal T and NKT cells (right panel). (G) Borrelia infection increases the frequency of NKTL. PTCL and NKTL frequency among all PTCLs in control and Borrelia-infected p53−/− mice with significantly different PTCL spectra. *P < .02, χ2 test.
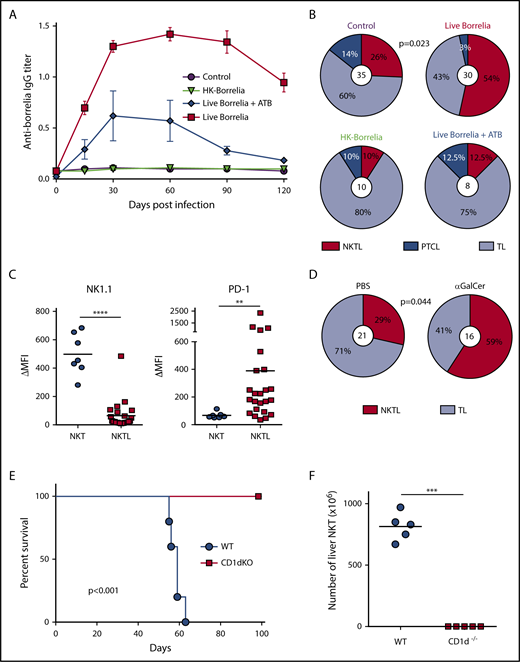
To further understand the role of chronic infection by Bb sl in NKTL development, we studied the incidence of NKTL under different experimental conditions, as well as the persistence of anti-Borrelia IgG, because these antibodies play an important role in immune responses against spirochetes.15 p53−/− mice infected with live B afzelii produced high levels of anti-Borrelia IgG that persisted for >120 days (Figure 2A), and they developed significantly more NKTLs compared with mice injected with Barbour–Stoenner–Kelly medium (Figure 2B). Conversely, mice injected with heat-killed (HK) Borrelia or Borrelia-infected mice treated with antibiotics to eradicate bacteria16 did not produce long-term persisting anti-Borrelia IgG (Figure 2A) and did not show any significant increase in NKTL frequency (Figure 2B). These results demonstrated the importance of live Borrelia persistence (ie, chronic infection) in NKTL development. NKTL showed downregulated NK1.1 and increased PD-1 expression, 2 characteristics of chronically activated NKT lymphocytes (Figure 2C). The role of chronic TCR stimulation in NKT lymphomagenesis was further demonstrated by the increase in NKTLs after chronic injections with αGalCer (Figure 2D).
NKTL is driven by chronic TCR activation. (A) Borrelia induces chronic infection in p53−/− mice. Kinetics of anti-Borrelia IgG titer in p53−/− mice sera by enzyme-linked immunosorbent assay. Mice (n = 8-10 per group) were injected intradermally with control medium, heat-killed (HK) Borrelia, or live Borrelia, treated (blue line) or not (red line) with ceftriaxone (antibiotics; ATB) at 25 mg/kg, twice a day for 5 days, starting 10 days postinfection, to eradicate the bacteria after short-term infection. Data are mean ± SEM. P = .031 for kinetics between live Borrelia and live Borrelia + ATB, Kruskal-Wallis test. (B) Chronic infection is required to increase NKTL frequency. Lymphoma spectrum developed in p53−/− mice injected with control medium, live Borrelia, HK Borrelia, or live Borrelia + ATB. Only live Borrelia injection induced significantly more NKTL compared with control mice (P = .023, χ2 test). Of note, the number of tumors exceeded the total number of mice because some animals had several tumors (ie, a solid tumor and a lymphoma). Values in the center refer to the total numbers of lymphomas. (C) NKTL show features of chronically activated NKT cells. Flow cytometric analyses of NK1.1 and PD-1 expression on NKTL, showing a significant loss of NK1.1 (****P < .0001) and overexpression of PD-1 (**P = .002), Mann-Whitney U test. (D) NKT-activating glycolipids drive NKTL. Lymphoma spectra developed in PBS- and αGalCer-injected p53−/− mice. Mice were injected intraperitoneally every week with PBS or 4 µg of αGalCer. αGalCer injection induced significantly more NKTL compared with PBS. The P value was determined using the χ2 test. (E) NKTL depend on TCR-CD1d interaction for engraftment and survival in vivo. Survival curves of WT and CD1d−/− mice (n = 5) injected IV with 106 NKTL cells, showing the absence of lymphoma development in CD1d−/− recipient mice. All mice alive 100 days postinfection were euthanized. The P value was determined using the log-rank test. (F) Number of total liver NKT cells at sacrifice. ***P =.008, Mann-Whitney U test. TL, thymic lymphomas.
NKTL is driven by chronic TCR activation. (A) Borrelia induces chronic infection in p53−/− mice. Kinetics of anti-Borrelia IgG titer in p53−/− mice sera by enzyme-linked immunosorbent assay. Mice (n = 8-10 per group) were injected intradermally with control medium, heat-killed (HK) Borrelia, or live Borrelia, treated (blue line) or not (red line) with ceftriaxone (antibiotics; ATB) at 25 mg/kg, twice a day for 5 days, starting 10 days postinfection, to eradicate the bacteria after short-term infection. Data are mean ± SEM. P = .031 for kinetics between live Borrelia and live Borrelia + ATB, Kruskal-Wallis test. (B) Chronic infection is required to increase NKTL frequency. Lymphoma spectrum developed in p53−/− mice injected with control medium, live Borrelia, HK Borrelia, or live Borrelia + ATB. Only live Borrelia injection induced significantly more NKTL compared with control mice (P = .023, χ2 test). Of note, the number of tumors exceeded the total number of mice because some animals had several tumors (ie, a solid tumor and a lymphoma). Values in the center refer to the total numbers of lymphomas. (C) NKTL show features of chronically activated NKT cells. Flow cytometric analyses of NK1.1 and PD-1 expression on NKTL, showing a significant loss of NK1.1 (****P < .0001) and overexpression of PD-1 (**P = .002), Mann-Whitney U test. (D) NKT-activating glycolipids drive NKTL. Lymphoma spectra developed in PBS- and αGalCer-injected p53−/− mice. Mice were injected intraperitoneally every week with PBS or 4 µg of αGalCer. αGalCer injection induced significantly more NKTL compared with PBS. The P value was determined using the χ2 test. (E) NKTL depend on TCR-CD1d interaction for engraftment and survival in vivo. Survival curves of WT and CD1d−/− mice (n = 5) injected IV with 106 NKTL cells, showing the absence of lymphoma development in CD1d−/− recipient mice. All mice alive 100 days postinfection were euthanized. The P value was determined using the log-rank test. (F) Number of total liver NKT cells at sacrifice. ***P =.008, Mann-Whitney U test. TL, thymic lymphomas.
Normal NKT cells persist in vivo in the absence of TCR/CD1d interactions.17 Conversely, during chronic infections, the survival of antigen-specific memory T cells relies on the persistence of cognate antigens through repeated TCR engagements.18 To investigate the importance of CD1d-mediated chronic TCR engagement in NKTL survival, we transferred NKTL cells into CD1d−/− recipient mice. All wild-type (WT) recipients succumbed to lymphoma, whereas all CD1d−/− mice remained healthy (Figure 2E). Liver cellularity increased significantly in WT recipient mice; NKTL cells represented 92% to 95% of total liver cells, whereas they were undetectable in CD1d−/− recipient mice (Figure 2F). Such dependence to CD1d/TCR interaction strongly suggests that NKTL development is driven by chronic TCR stimulation.
In summary, we described for the first time that chronic Bb sl infection increases the incidence of PTCL originating from NKT cells in p53−/− mice. These results reinforce previous data demonstrating the role of TCR in NKT lymphomagenesis.13 In addition to transforming viruses, such as Epstein-Barr virus19 and human T-cell leukemia virus, type 1,20 which are implicated in several non-Hodgkin lymphoma subtypes, chronic immune stimulation by pathogens, such as H pylori3 and hepatitis C virus,21 has been reported to trigger lymphomagenesis. This study constitutes a novel illustration of the association among bacterial infections, chronic antigen receptor stimulation, and lymphoma development, as well as a formal demonstration that Bb sl infection drives T-cell lymphomagenesis. Whether Bb infection also triggers NKTL in humans needs to be further investigated, because our p53−/− model may not recapitulate the pathophysiological genetic events of mycosis fungoides or other PTCLs associated with B burgdorferi infection. However, recent data from whole-exome sequencing have identified TP53 mutations and copy number alterations as the most prevalent genetic abnormalities in cutaneous T-cell lymphoma,22,23 suggesting that TP53 alterations might be the genetic driver events associated with chronic TCR stimulation in cutaneous T-cell lymphomagenesis associated with Borrelia infection. In addition, PLZF, a key transcription factor in NKT cell differentiation, is overexpressed in some human T-cell lymphomas/leukemias, such as MF and Sézary syndrome (SS),24,25 suggesting that at least some SSs and/or MFs may be NKTLs.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the National Institutes of Health Tetramer Facility for mouse CD1d tetramers; N. Aguilera, J.-F. Henry, and P. Manas (PBES, SFR Biosciences Gerland - UMS3444/US8) for assistance in the animal facility; and Elody Collin (EA 7290) and Danièle Napolitano (National Reference Center for Borrelia) for technical assistance with Borrelia experiments.
L.G. was supported by Plan Cancer DESP/CG/SW no. 197, The Institut Carnot CALYM granted by the French National Research Agency, La Ligue Nationale Contre le Cancer–Equipe Labéllisée Ligue Contre le Cancer and La Ligue Contre le Cancer–comité du Rhône.
Authorship
Contribution: R.R. designed and performed experiments, analyzed data, created figures, and wrote the manuscript; S. Carras, M.U., S. Chaubard, E. Bardel, and D.C. performed experiments and analyzed data; E. Bachy, A.T.-G., P.N.M., G.S., and B.J. designed experiments and analyzed data; and L.G. designed research, analyzed data, created figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurent Genestier, Centre de Recherche en Cancérologie de Lyon, INSERM U1052, Faculté de Médecine Lyon-Sud, 165, Chemin du petit Revoyet, BP 12, 69921 Oullins Cedex, France; e-mail: laurent.genestier@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal