Abstract
Background:
JAK1/2 inhibitor ruxolitinib (RUX) abrogates symptoms and organomegaly in patients with myelofibrosis (MF). Combination with azacitidine (AZA) may further improve its efficacy.
Methods:
We initiated a single institutional, single arm, prospective, phase 2 study of RUX AZA combination in adult patients with MF and < 20% blasts. Previous therapy with RUX or AZA was not allowed. RUX 5 - 20 mg orally twice daily was given continuously since cycle 1. AZA 25 - 75 mg/m2 on days 1 - 5 of each 28-day cycle was added starting cycle 4. Responses were assessed per International Working Group for Myelofibrosis Research and Treatment 2013 criteria (IWG-MRT). Enrollment cut-off for this analysis was December 31st, 2017 to allow > 6 months of follow-up for all enrolled patients. We plan to present updated results with additional 5 months of enrollment at the meeting.
Results:
Fifty two pts were enrolled on study between 03/2013-12/2017, and were evaluable for responses. Forty seven pts (84%) were treated with both agents (RUX and AZA), with a median of 25 cycles (range, 1-55). Median age was 66 years (range, 48-87). Thirty four pts (65%) had int-2/high DIPSS score, 40 pts (77%) had spleen ≥5 cm. Thirty pts (58%) were JAK2V617F positive. Among 36 pts tested for non-driver mutations (28-gene panel); 7 pts had ASXL1, 6 had TET2, 3 had IDH1/2 and 2 had EZH2 and TP53.
After a median follow-up of 22+ months (range, 1-59+); 21 pts (40%) are on therapy with a median overall follow-up of 30+ months. The most common reasons for therapy discontinuation were elective stem cell transplantation (n=12), and uncontrolled disease (n=8), including progression to acute leukemia (n=4). Four pts (8%) primarily discontinued therapy due to drug related toxicity (cytopenias). Three treatment unrelated deaths occurred on study; one each due to sepsis, meningitis and metastatic melanoma.
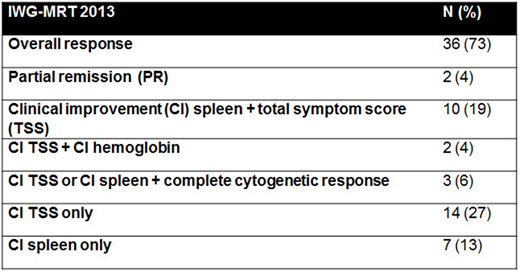
Thirty eight pts (73%) had objective response on a study (Table). Median time to response was 1.8 months (range, 0.7-19). Seven responses (21% of responders) occurred after the addition of AZA with a median time to response of 2 months. These responses included spleen and symptom clinical improvements in 26% and 16% of pts, respectively.
In total, 26 (65%), and 23 (58%) pts had palpable spleen reduction by > 50% at any time on study, and at week 24, respectively. JAK2V617F allele reduction was noted in 13 (81%) of 16 evaluable pts.
Thirty one pts (60%) had available bone marrow for sequential evaluation. Nineteen pts (61%) had a documented improvement in bone marrow fibrosis, collagen or osteosclerosis, with a median time to first response of 12 months (range, 6-18).
The most common grade ≥3 non-hematologic toxicity on a study was infection (34%), constipation (21%), and nausea (14%). New onset of grade ≥3 anemia, thrombocytopenia and neutropenia occurred in 33%, 30% and 16% of pts, respectively.
Conclusion:
Concomitant RUX with AZA was feasible with overall IWG-MRT response rate of 73%, including >50% spleen reduction in 65% of pts. Moreover, 61% of pts achieved improvement in bone marrow fibrosis, collagen or osteosclerosis. ClinicalTrials.gov Identifier: NCT01787487.
Table.
Verstovsek:Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Italfarmaco: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Cortes:novartis: Research Funding. Pemmaraju:novartis: Research Funding; daiichi sankyo: Research Funding; Affymetrix: Research Funding; plexxikon: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; SagerStrong Foundation: Research Funding. Bose:Blueprint Medicines Corporation: Research Funding; Incyte Corporation: Honoraria, Research Funding; Constellation Pharmaceuticals: Research Funding; Astellas Pharmaceuticals: Research Funding; Pfizer, Inc.: Research Funding; CTI BioPharma: Research Funding; Celgene Corporation: Honoraria, Research Funding. Daver:Pfizer: Consultancy; Novartis: Research Funding; ImmunoGen: Consultancy; Alexion: Consultancy; Incyte: Consultancy; Karyopharm: Research Funding; Sunesis: Research Funding; Otsuka: Consultancy; Novartis: Consultancy; Karyopharm: Consultancy; Sunesis: Consultancy; Daiichi-Sankyo: Research Funding; ARIAD: Research Funding; Incyte: Research Funding; Kiromic: Research Funding; Pfizer: Research Funding; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal