Abstract
Introduction
TP53 mutations in relapsed cases with pediatric acute lymphoblastic leukemia have been implicated in poor clinical outcomes. However, the prevalence and clinical significance of TP53 mutations at diagnosis have not been fully investigated. Such knowledge is essential for the care of patients, because treatment intensity is tailored to predictive prognosis, where increased attention has been directed toward de-escalation of treatment for the problem of long term effects and second malignancies in childhood cancer survivors.
Methods
Mutation status of TP53 was detected by targeted-capture sequencing of TP53 coding regions in 1,003 children with B-precursor ALL who had been treated in either of the two prospective clinical trials, JACLS (Japan Association of Childhood Leukemia Study) ALL-02 and TCCSG (Tokyo Children's Cancer Study Group) L04-16. Detection of common fusion genes, including BCR-ABL, ETV6-RUNX1, MLL-AF4, MLL-ENL, MLL-AF9, and TCF3-PBX1, were performed using qPCR assays. We designed SNP baits to analyze copy number status of chromosome 17, and also captured 662 probes tiling the entire IgH enhancer locus to identify IGH-DUX4 rearrangement.
Result
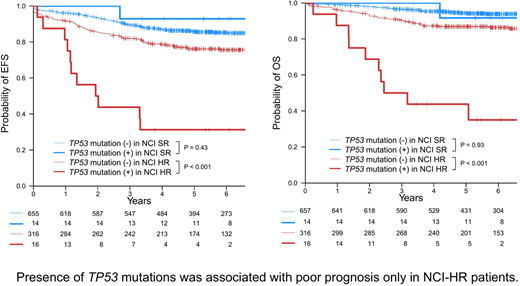
In total, 36 different non-silent coding TP53 mutations were identified in 30 (3%) patients, including 22 missense, 7 frameshift indel, 5 in-frame indel, and 2 nonsense mutations. All missense mutations were found in the core DNA-binding domain (n=21), except for one mutation, which affected the tetramerization motif. Variant allele frequencies (VAF) of TP53 mutations varied from 3% to 97% with 14 mutations showing < 10% VAFs. Showing a significant correlation with mutated TP53 (Odds ratio 20: 95%CI 6.4-61, P<0.001), loss of heterozygosity affecting the TP53 locus was observed in 11 (37%) cases and caused by del(17p) in most cases (n=10). We next evaluated clinical features of TP53-mutated cases. TP53 was most frequently mutated in Hypodiploid ALL (33% n=3), followed by MLL rearrangement (12% n=4), IGH-DUX4 (9% n=5), Others (3% n=8), TCF3-PBX1 (2% n=2), Hyperdiploid (2% n=6), and ETV6-RUNX1 (n=2 0.9%). TP53 mutations were not associated with age or white blood cell count at diagnosis. However, significantly more patients were categorized into National Cancer Institute (NCI) high risk (HR) category (Odds ratio 2.4: 95%CI 1.1-5.3, P = 0.03) and TP53 mutation was associated with a significantly shorter overall survival (OS) among NCI-HR patients (n = 16; HR for death, 6.3; 95% CI, 3.1-13; P<0.001). Five-year OS of NCI-HR patients with TP53 mutations was 44%, suggesting that early treatment intensification or alternative treatment strategies are warranted for these patients. TP53 mutations were also associated with a shorter OS in MLL rearrangement and IGH-DUX4 ALL. Particularly, 67% (n=4/6) of cases with any cause of death harbored TP53 mutation in IGH-DUX4 ALL. In contrast, TP53 mutation was not associated with shorter overall survival in NCI-SR cases. In Hyperdiploid ALL, 5 out of 6 cases with TP53 mutations were categorized into the NCI-SR category and were all alive. Prognostic impact of TP53 mutation was also investigated using recursive partitioning to generate a hierarchical prognostic model for OS by incorporating genetic subgroups and the NCI risk criteria. This model also demonstrated that the NCI risk criteria was the most important prognostic variable and TP53 mutation was used for stratification of patients only in the NCI-HR category.
Conclusion
TP53 mutations at diagnosis are common in Hypodiploid ALL and also found in a substantial fraction of MLL rearrangement and IGH-DUX4 ALL, where the mutations predict a poor prognosis. TP53 mutation is also found in NCI-SR cases but may not be associated with poor prognosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal