Abstract
The natural history of FLT3-mutated AML is changing after the approval of midostaurin for frontline therapy and gilteritinib for relapsed or refractory patients. Recently reported, positive randomized trials of the drugs gilteritinib, quizartinib, and sorafenib predict even wider use of FLT3 inhibitors going forward. FLT3 inhibitors now emerge as an important, if not indispensable, part of therapy for a large subset of high-risk patients.
Introduction
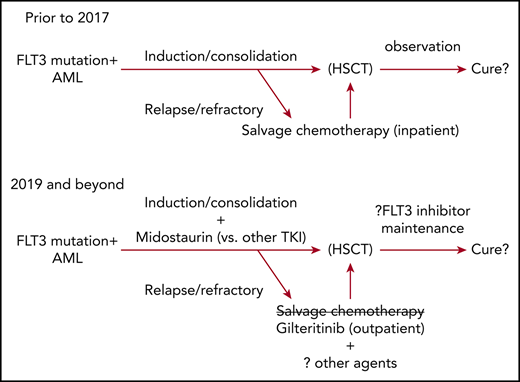
On April 28, 2017, and November 28, 2018, the US Food and Drug Administration approved 2 drugs for the therapy of patients with AML with FLT3 mutations (FLT3mut+). First, midostaurin (Rydapt, Novartis Pharmaceuticals Corp.) was approved for newly diagnosed FLT3mut+ AML in combination with daunorubicin and cytarabine induction and consolidation, based on improved overall survival (OS) reported from the CALGB10603 (RATIFY) study.1,2 More recently, gilteritinib (Xospata, Astellas Pharma US, Inc.) was approved for relapsed or refractory FLT3mut+ AML, based on response rates from a phase 3 trial’s interim analysis (ADMIRAL, NCT02421939)3 ; subsequently, a survival benefit for gilteritinib over salvage chemotherapy from this study’s final analysis was reported.4 After decades of therapeutic stagnation, these approvals have helped usher AML into an era of molecularly targeted therapy for a large subset of patients.
FLT3 mutations occur in approximately 30% of newly diagnosed AML and are relatively more common in young and middle-aged adults with normal or intermediate-risk karyotype.5,6 Among FLT3mut+ patients, 3/4 have internal tandem duplications (FLT3-ITD) and 1/4 missense mutations within the tyrosine kinase domain (TKD) activation loop, usually at codon D835. Both mutations cause constitutive kinase activation, contribute to cellular transformation in vitro, and clinically are associated with leukocytosis and aggressive, proliferative disease.7,8 FLT3-ITD is a strong risk factor for relapse, particularly in the presence of DNMT3A mutation, the absence of NPM1 mutation, or when a high ITD allele burden is present at diagnosis, and shows inferior OS compared with FLT3-TKD or wild-type FLT3.5,6,9-13 For these reasons, FLT3-ITD+ patients are frequently referred for early hematopoietic stem cell transplantation (HSCT), and efforts to improve therapeutic outcomes via FLT3-targeted tyrosine kinase inhibitors have long been sought.
Multikinase inhibitors for FLT3mut+ AML
Nearly all the initial agents tested for activity as FLT3 inhibitors were broadly kinase-inhibitory drugs, and none was specifically designed as a FLT3 inhibitor per se. Early-phase testing of midostaurin as a single agent in relapsed/refractory patients showed the drug reduced or eliminated circulating blasts in FLT3mut+ or FLT3-wild-type patients, but had limited effect on the marrow blast content and a short median therapeutic duration.14 Therefore, subsequent midostaurin clinical development was limited to combination regimens, where it ultimately proved more successful.
CALGB10603/RATIFY showed midostaurin resulted in a 22% reduction in mortality risk for newly diagnosed FLT3mut+ patients when added to intensive induction, consolidation, and postconsolidation maintenance.2 This translated into a 7% absolute improvement in estimated OS at 4 years from diagnosis (51.4% vs 44.3% for midostaurin and placebo, respectively); survival benefits were seen without regard to the type of FLT3 mutation (ITD or TKD) or the allele burden of FLT3-ITD. Although this randomized study only enrolled patients younger than 60 years, the feasibility of intensive chemotherapy and midostaurin in fit older patients was recently shown by a single-group German cooperative group trial.15 Although midostaurin became the first drug specifically approved for FLT3mut+ patients, the leukemia community already had gained experience with FLT3 inhibitors through off-label availability of sorafenib. Unlike single-agent midostaurin, complete remissions (CR), often without full hematologic recovery (CRi) were seen in 43% to 70% of patients treated with sorafenib plus azacitidine, and durable CR was seen in 17% of patients treated with sorafenib alone for post-HSCT relapse.16-18 In addition, single-center reports suggested a benefit of sorafenib as post-HSCT maintenance.19,20 The recently reported randomized, placebo-controlled phase 2 SORMAIN study supports this approach, showing a substantial reduction in relapse (hazard ratio, 0.39; P = .013), as well as an similar OS benefit from 2 years of post-HSCT sorafenib maintenance.21 Although promising, sorafenib can be associated with significant toxicity, including hand-foot syndrome, gastrointestinal irritation, asthenia, cytopenias, and an increased risk for serious infections, which potentially increase induction mortality.22,23 Therefore, in the absence of a proven OS benefit, sorafenib has not seen widespread use in frontline intensive chemotherapy.
Selective/potent FLT3 inhibitors
Gilteritinib is the first FLT3 inhibitor to be approved for single-agent use, but this drug is one of a series of agents designed to address shortcomings of prior FLT3 inhibitors. Of these, quizartinib likely played the most important role in establishing single-agent FLT3 inhibition as a therapeutic strategy. Quizartinib was selected for clinical development on the basis of its narrow tyrosine kinase inhibition spectrum, which was hoped to maximize FLT3 inhibition and minimize off-target adverse effects.24 Seminal correlative studies by Mark Levis at Johns Hopkins demonstrated that the in vivo FLT3 inhibitory potency of drugs such as midostaurin, lestaurtinib, KW-2449, and sorafenib were generally weak or not sustained, and clinical activity was limited to patients who achieved prolonged plasma drug levels that profoundly inhibited FLT3 kinase in a cellular assay (plasma inhibitory activity, reviewed in Levis et al25 and Pratz, et al26 ). Unlike prior agents, in which profound inhibition meant reduction in residual FLT3 phosphorylation to less than 15% of baseline, early quizartinib studies consistently demonstrated sustained FLT3 kinase abrogation by plasma inhibitory activity.27 Quizartinib therapy also cleared circulating blasts and reduced marrow blasts to less than 5% in 46% to 53% of relapsed/refractory FLT3-ITD+ patients, which allowed up to 35% of treated patients to subsequently receive HSCT.27-29
However, clinical responses to quizartinib, and indeed other potent, selective FLT3 inhibitors, qualitatively did not resemble those of cytotoxic chemotherapy. Quizartinib responses primarily were not CR but, rather, were CR with incomplete count recovery (CRi), using a modified international working group response definition that allowed both residual neutropenia and thrombocytopenia.27,30-32 A substantial number of responders had partial remissions (PR), which were also defined more loosely than by international working group criteria.27,30-32 Therefore, although overall response rates of 50% to 70% from selective, potent FLT3 inhibitor trials in relapsed/refractory FLT3mut+ patients were encouraging,27,28,30,31,33 gauging the clinical benefit of these drugs has been challenging before phase 3 data availability. The lack of modified CRi or PR response validation certainly slowed quizartinib’s clinical development, as did initial selection of phase 2 doses that were considerably higher than necessary to inhibit FLT3 potently. This contributed to frequent QT prolongation and myelosuppression,27,28 necessitating subsequent quizartinib studies at lower doses. This showed equivalent efficacy but improved tolerability, facilitating phase 3 initiation.33
The QuANTUM-R trial randomized 367 FLT3-ITD+ patients with primary refractory leukemia or an early relapse (<6 months from first remission or transplant) to single-agent quizartinib vs investigator’s choice of salvage chemotherapy (SC): fludarabine, cytarabine, filgrastim, idarubicin (FLAG-IDA), mitoxantrone, etoposide, cytarabine (MEC), or low-dose cytarabine.29 The study’s primary end point of OS was statistically significantly longer in the quizartinib group than SC (6.2 vs 4.7 months, respectively; P = .0177), and 27% of the quizartinib group and 20% of SC patients were alive at 1 year. It is notable that SC response rates were quite low: only 1% achieved full CR or CRp (CR with incomplete platelet recovery), and only 30% had any protocol-defined response of CR, CRp, CRi (so called “composite CR,” CRc), or PR. Similarly, only 8% of quizartinib-treated patients reached CR or CRp, but 69% had a CRc or PR. Transplant occurred more frequently among quizartinib (32%) than SC (12%), and 62% of transplanted quizartinib group patients received post-HSCT quizartinib maintenance. With the exception of QT prolongation (4% grade 3 on the quizartinib group with no QT-related arrhythmias), common treatment-emergent adverse events were less frequent among quizartinib group patients than SC.29 Although the US Food and Drug Administration’s decision regarding approval based on the QuANTUM-R data are pending, its Oncology Drug Advisory Committee recently recommended against approval, citing both toxicity concerns and marginal clinical benefit, particularly in comparison with more intensive salvage options.
A concerning weakness of both quizartinib and sorafenib therapy of relapsed/refractory patients has been the frequent and rapid selection for clones with on-target mutational drug resistance, including mutations in the FLT3-TKD activation loop (D835) and/or gatekeeper residues (F691) with preserved FLT3-ITD.34,35 These data clarified that clinical benefits from potent/selective FLT3 inhibitors were unlikely to be maximized unless a drug could be developed that was a potent, selective inhibitor of both FLT3-ITD and these resistance mechanisms.
Two promising agents have emerged to address these issues: crenolanib and gilteritinib, which as type 1 kinase inhibitors have the ability to more broadly inhibit FLT3 in the presence of TKD mutations.36-38 Both showed potent, continuous target inhibition by plasma inhibitory activity and substantial single-agent activity in relapsed/refractory patients with FLT3-ITD, FLT3-D835, or both mutations.30,31 Pharmacokinetic properties (thrice-daily dosing) have primarily steered crenolanib development toward multiagent regimens, but once-daily gilteritinib has been developed as a single agent for relapsed and refractory FLT3mut+ patients. In this context, gilteritinib has realized essentially all goals for a FLT3 inhibitor in terms of its selectivity for FLT3: very modest adverse effects (eg, asymptomatic hepatic transaminase or CPK elevation, edema, and cytopenias) and single-agent clinical activity against both FLT3-ITD and FLT3-D835 resistance mutations.30 Gilteritinib resistance mutations have been characterized, but lack on-target mutations other than an approximately 10% incidence of FLT3-F691L.39
Phase 3 testing of gilteritinib (ADMIRAL) in primary refractory or untreated first relapse of FLT3mut+ AML used a study design that was largely overlapping with QuANTUM-R’s. Gilteritinib was associated with superior median survival as compared with salvage chemotherapy (9.3 vs 5.6 months, respectively; P = .0007), and exposure-adjusted incidences of nearly all adverse events, including serious adverse events, were less frequent with gilteritinib.4 Notable exceptions were hepatic transaminase elevations, which occurred more commonly with gilteritinib. Eight-week transfusion independence during therapy occurred in 39% of gilteritinib treated patients (35% of those transfusion dependent at baseline). Similar to QuANTUM-R, long-term survival was uncommon in both ADMIRAL groups, and was largely predicted by receipt of transplant. Interestingly, survival remained improved by gilteritinib even when ADMIRAL’s data were censored for HSCT. An exploratory analysis in the gilteritinib arm suggested that long-term posttransplant survival was primarily seen among patients who restarted the FLT3 inhibitor as posttransplant maintenance; this result should be cautiously interpreted because of competing contributors to mortality risk that affect maintenance use.
Putting the data together: using FLT3 inhibitors in 2019
Midostaurin and gilteritinib are now approved for FLT3mut+ AML, sorafenib remains available off-label, and quizartinib is under US Food and Drug Administration review. Although much is still to be learned about how best to use FLT3 inhibitors, an important takeaway is that FLT3 inhibitors are an integral part of intensive frontline induction and salvage therapy based on evidence from prospective, randomized controlled trials in each scenario. Rarely have we had such compelling data to support therapy choice in AML as we have now for this class of drugs.
The final ADMIRAL results demonstrate gilteritinib’s merits relative to salvage chemotherapy, but do not show that the sinister natural history of relapsed/refractory FLT3-mutated AML is eliminated via single-agent targeted therapy. Still, gilteritinib is a reasonable and well-tolerated approved, outpatient therapeutic option that can provide long-term benefit, especially among patients who bridge to transplant and receive post-HSCT maintenance. Gilteritinib is active in patients who are resistant to quizartinib or sorafenib by virtue of FLT3-D835, but whether one should sequence gilteritinib first or only after these agents fail is unknown. Because a gatekeeper residue in FLT3 or treatment-emergent mutations in parallel (MAPK) signaling can confer pan resistance to all FLT3 inhibitors, my general recommendation is to start with gilteritinib, given its availability, activity in both ITD and D835 mutations, and tolerability paired with a relatively long duration of response and proven superior survival in clinical trials compared with standard chemotherapy. The exceedingly poor performance of the salvage chemotherapy groups from either QuANTUM-R or ADMIRAL cytotoxic chemotherapy make it hard to recommend traditional chemotherapy alone as a first salvage choice. But whether adding potent FLT3 inhibitors to salvage chemotherapy improves activity is an open question that is currently being studied in clinical trials (NCT03250338).
To date, only midostaurin has shown a survival benefit over placebo in frontline treatment and, despite exciting data with newer and more potent FLT3 inhibitors,40,41 it is wholly unknown whether these drugs indeed will prove superior to midostaurin’s performance in this context. Studies comparing gilteritinib or crenolanib with midostaurin to identify the optimal FLT3 inhibitor to be combined with 7+3 are ongoing (NCT03258931, NCT03836209) or planned.
Transplant remains an important part of therapy for many FLT3-ITD+ patients. FLT3 inhibitors appear to improve post-HSCT survival, whether given only before first remission transplant (eg, midostaurin on C10603/RATIFY)2 or when used as post-HSCT maintenance only (eg, sorafenib on SORMAIN).21 Whether FLT3 inhibitors and following HSCT further improve survival is unproven, although a landmark analysis of patients treated with midostaurin both before and as maintenance after HSCT on the single-group AMLSG 16-10 study showed reduced post-HSCT relapses relative to historic controls.15 The absence of frontline midostaurin therapy on both SORMAIN as well as a randomized, posttransplant midostaurin maintenance trial limits generalizability of these studies’ results to current therapy.21,42 Perhaps only a subset, such as those who have measurable residual disease pre-HSCT, derive benefit from maintenance FLT3 inhibitors. Given the uncertain role of maintenance with current treatment approaches, as well as toxicity noted on both posttransplant sorafenib and midostaurin studies,15,21,42 placebo-controlled trials of more potent FLT3 inhibitors given as maintenance after CR1 HSCT are ongoing (NCT02997202).43
Clinicians and hematopathologists need to gain familiarity with the different patterns of response to salvage therapy with potent, selective FLT3 inhibitors. Incomplete peripheral count recovery occurs frequently among responding patients, and full peripheral reconstitution is not prerequisite for prolonged post-HSCT survival.28 Therefore, residual cytopenias generally should not be a roadblock to transplant. Similar to isocitrate dehydrogenase inhibitors, selective FLT3 inhibitors can induce terminal differentiation of blasts to neutrophils.44-46 Accordingly, changes in marrow FLT3 mutation burden also should not be used to determine transplant candidacy, as mutational clearance is uncommon. In some patients, noninfectious fevers, Sweet’s syndrome-like rashes, arthritis, edema/fluid retention, or serositis potentially indicate manifestations of a differentiation syndrome to potent, selective FLT3 inhibitors.44,46-48 Although not rigidly defined on trials, such symptoms anecdotally occur in up to 10% of patients, often coincide with rising neutrophil counts after 3 to 4 weeks of treatment, and can be ameliorated by topical or systemic corticosteroids when symptomatic.44
Eligible relapsed/refractory patients responding to FLT3 inhibitor salvage should be transplanted expeditiously. It seems prudent to proceed to HSCT once a marked reduction in marrow blasts is achieved, typically after 1 and 2 months of FLT3 inhibitor therapy. For relapsed/refractory patients successfully salvaged and bridged to transplant with FLT3 inhibitors, post-HSCT FLT3 inhibitor maintenance should be encouraged and initiated early, if feasible (eg, day +30). This recommendation follows observational evidence of high posttransplant relapse rates without maintenance and rapid kinetics.4,28
Conclusion
FLT3 inhibitors are finally standard therapy for newly diagnosed and relapsed/refractory patients with FLT3mut+ AML. An important goal now is to define effective, tolerable combination regimens, and move these to frontline therapy, where they hopefully will prevent relapse. With the panoply of recently approved novel agents for AML, there are unprecedented opportunities to define new treatment approaches. Hopefully, the optimization of targeted, combination approaches will one day relegate the sinister implications of FLT3-ITD to historic interest, much as has occurred in acute promyelocytic leukemia.
Authorship
Contribution: A.E.P. wrote the manuscript
Conflict-of-interest disclosure: A.E.P. reports consultancy or advisory board membership for the following: AbbVie, Agios, Takeda, Astellas, Daiichi Sankyo, Arog, Novartis, NewLink Genetics, Actinium Pharmaceuticals, and Pfizer.
Correspondence: Alexander E. Perl, 12-154 South Tower, Perelman Center for Advanced Medicine, Division of Hematology/Oncology, Perelman School of Medicine at the University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: alexander.perl@uphs.upenn.edu.