Abstract
Patients with hematologic malignancies and those undergoing hematopoietic cell transplantation (HCT) face a complex set of challenges when considering options for fertility preservation (FP). There are no standard options for prepubertal children, and women with hematologic malignancies may not be eligible for standard FP options. Fortunately, initial therapies for most blood cancers are not highly gonadotoxic, affording an important opportunity for postremission counseling and referrals to fertility specialists. These patients face a high risk of relapse, and many will be referred for autologous or allogeneic HCT, which carries an extremely high risk of infertility. The expanding indications for HCT to include benign hematologic disorders as well as autoimmune diseases mandate that all hematologists are familiar with these risks. Oncofertility researchers are continually pushing the boundaries of what may be possible for our patients; in the meantime, communication and shared decision-making between hematologists and patients, as well as program-building, education, and outreach are essential to ensure that these patients, many of whom will be cured, maintain all of their options for a fulfilling life after intensive therapy.
In 2016, there were 15.5 million cancer survivors in the United States, projected to increase to 20 million in 2026. About 7% to 8% will be survivors of a hematologic malignancy.1 For children and adolescents with hematologic malignancies, 5-year survival is high: 60% for acute myeloid leukemia, to nearly 90% for acute lymphoblastic leukemia, and 98% for Hodgkin lymphoma (HL). Younger adults also enjoy good outcomes, and death rates are declining.1 There will be approximately 242 000 survivors of hematopoietic cell transplant (HCT) by 2020, which accounts for roughly 1% of all survivors.2
The reproductive system is among the most vulnerable to damaging sequelae of cancer and its treatments; patients also experience significant psychosocial and financial harms.3,4 This review is the first to focus on the unique fertility preservation (FP) concerns faced by patients with hematologic malignancies and those undergoing HCT. Existing guidelines for FP, such as those from the American Society for Reproductive Medicine,5 the National Comprehensive Cancer Network,6 and the American Society for Clinical Oncology7,8 address the general needs of all people with cancer, relying mainly on research in patients with breast and testicular cancer. There has been 1 clinical guide to FP in patients undergoing HCT.9 Patients with hematologic malignancies demand special consideration because (1) blood cancers such as acute lymphoblastic leukemia and HL are significantly more common in children and young adults, putting FP front and center; (2) patients with blood cancers may present with medical complications that preclude standard FP options; (3) some FP techniques are contraindicated in patients with hematologic malignancies; (4) sometimes up front, and nearly always at relapse, these patients are offered HCT, which is associated with almost universal infertility. Hence, there is a significant unmet need to address FP in the unique circumstances faced by hematology patients. This review will address FP for patients with acute leukemias, HL and non-HL, chronic myeloid leukemia (CML), and those receiving HCT. Increasingly, HCT is considered in the management of some nonmalignant conditions, including sickle cell disease (SCD) and autoimmune diseases; all hematologists should therefore be familiar with these issues.
Concern about infertility is significant in survivors of cancer and contributes to distress and poor quality of life
Infertility is common after cancer treatment. Compared with their siblings, male cancer survivors are twice as likely to experience infertility10 and 44% less likely to father a child11 ; female survivors are 18% less likely to report a live birth12 (Figure 1). Survivors of HCT are 36-fold less likely to conceive a pregnancy.13
Cumulative incidence of pregnancies and livebirths in cancer survivors and siblings. Incidence curves are shown with upper and lower 95% confidence intervals. (A) Male cancer survivors, first ever pregnancy sired (left) and first ever livebirth sired (right). (B) Female cancer survivors, first ever pregnancy (left) and first ever livebirth (right). Reprinted from Chow et al12 with permission from Elsevier.
Cumulative incidence of pregnancies and livebirths in cancer survivors and siblings. Incidence curves are shown with upper and lower 95% confidence intervals. (A) Male cancer survivors, first ever pregnancy sired (left) and first ever livebirth sired (right). (B) Female cancer survivors, first ever pregnancy (left) and first ever livebirth (right). Reprinted from Chow et al12 with permission from Elsevier.
The prospect of infertility looms large,14-17 and distress about infertility and regret about lack of information can lead to depression and diminished quality of life.15,18-20 Decision regret (regret after patient treatment-related decisions)21 can be reduced by pretreatment counseling,22 yet physicians inconsistently provide information to patients about fertility risks. Up to 68% of patients report that fertility concerns were not discussed as part of their conversations about therapy.23-25 Collaborative care pathways can significantly increase referrals to reproductive specialists.26-30 Counseling and engagement in FP reduce reproductive concern31 . Sociodemographic disparities have been identified: women are referred to reproductive specialists less frequently than men32 and patients who are white, childless, educated, heterosexual, and privately insured are more likely to be counseled and referred.24,33,34 Simply having a discussion about infertility risks can lower distress and improve quality of life after treatment.18,35
Chemotherapy and radiation may compromise fertility in both men and women, but the biology and long-term effects of cancer therapy differ between sexes
Women are born with all of the oocytes they will ever have. The number of oocytes peaks at approximately 7 million in the female fetus at 20 weeks’ gestation. The ovarian follicles undergo a steady process of atresia, falling to about 1 million at birth and continuing to decline until menopause, when the “stockpile” of oocytes is depleted.36,37 This fixed number of oocytes in women stands in contrast to the virtually unlimited number of sperm produced by men (provided that spermatogonial stem cells are present). Sperm are continually produced in the walls of the seminiferous tubules beginning in puberty at the rate of 45 to 270 million sperm per day38 ; sperm production takes approximately 74 days.39
Men
Low sperm counts are common in men with cancer even before the initiation of therapy. Although best described in men with testicular cancer, men with HL and leukemia40,41 are also affected. The testes are exquisitely sensitive to damage by cytotoxic treatments resulting from the prolific and continual production of mature spermatozoa. Oligo- and azoospermia may result from damage to the Sertoli and Leydig cells, toxicity to the stem spermatogonia, and damage to the hypothalamic-pituitary-gonadal axis.42-45 Mature sperm are relatively resistant to depletion, but sensitive to DNA damage and therefore unsuitable for sperm banking if recently exposed to cytotoxic therapy.46 This highlights the importance of effective contraception from the very beginning of treatment.
Alkylating agents, platinum, and radiation are most toxic to sperm production. Age at the time of treatment does not appear to affect the risk of infertility in adults, but the impact of age at treatment in prepubertal boys is unknown.44,47 Permanent azoospermia occurs in >90% of men with HL after treatment with alkylator-intensive regimens such as mustargen, vincristine, procarbazine, and prednisone (MOPP) and cyclophosphamide, vincristine, procarbazine, and prednisone (COPP), but doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) results in only transient oligozoospermia with return to normal sperm counts in about 18 months.48 Estimating intensity of therapy with a model using cyclophosphamide equivalents can reliably predict fertility risk.49
Radiation has complex effects on spermatogenesis. Even low doses of radiation (<0.1 Gy) can impair sperm production. Temporary azoospermia can occur after doses as low as 2 to 3 Gy; 4 to 6 Gy or more results in high rates of azoospermia. The likelihood of recovery becomes less likely with higher doses: patients receiving >6 Gy are likely to be permanently azoospermic.50 Oligozoospermia occurs approximately 10 weeks after radiation exposure and azoospermia at about 18 weeks.51 Fractionated therapy accentuates toxicity.52 After myeloablative (MA) conditioning before HCT, men nearly universally demonstrate hypergonadotropic hypogonadism and azoospermia.
Over time, sperm counts may recover: up to 50% of men may experience a return of spermatogenesis, varying from 12 to 18 months after treatment to 5 years or more, depending on the intensity of therapy.53 Recovery may occur even after high-dose alkylating agents and total body irradiation at 12 Gy.52,54 Because recovery is variable, expert panels suggest yearly surveillance for impaired spermatogenesis, testosterone deficiency, and sexual dysfunction.50 Graft-versus-host disease is an independent risk factor for prolonged azoospermia after autologous or allogeneic HCT (alloHCT), imparting a twofold increased risk.55
Women
It is difficult to measure fertility reliably in women. Amenorrhea is not equivalent to infertility, and conversely, women who resume cyclical menses may not have normal fertility. A better endpoint is ovarian reserve, an assessment of the quantity of oocyte-containing follicles. Estradiol, follicle-stimulating hormone, luteinizing hormone, and cyclical menses are poor measures of ovarian reserve; anti-Müllerian hormone (AMH) levels56-58 and antral follicle counts (AFC) via transvaginal ultrasound may be more accurate.59 Women do not produce additional gametes and recovery of ovarian function does not occur. A common misperception is that if patients resume normal menstrual function after cancer therapy, their ovarian reserve is intact. In fact, premature ovarian failure (POF) is a common outcome even in women who resume menses after treatment.60-62 POF is one of the most important, and underrecognized,61 consequences of cytotoxic therapy, occurring from the depletion of primordial follicles and resultant diminution of ovarian reserve.61,63 In a large study of survivors of childhood cancer who were menstruating at age 21, the risk of menopause by age 25 was increased 3.6-fold in women who received abdominal radiation, 9.2-fold with alkylator-based chemotherapy, and 27-fold in women who received both.64 These findings have been confirmed.62,65,66
The chance of permanent amenorrhea is related to the patient’s age, chemotherapeutic drug class, and dose. Alkylating agents, especially procarbazine and busulfan, and platinum confer an excess risk of POF.67 Older women are less likely to resume menses after treatment68 (Figure 2) and if menses do resume, POF is more likely to ensue, with a 23% increased risk of POF per year of age at treatment.69
Likelihood of women treated for breast cancer resuming menses after chemotherapy based on age at the time of therapy. Reprinted from Petrek et al68 with permission.
Likelihood of women treated for breast cancer resuming menses after chemotherapy based on age at the time of therapy. Reprinted from Petrek et al68 with permission.
There are a number of mechanisms by which chemotherapy may affect ovarian health. Because chemotherapy preferentially damages dividing cells, maturing follicles are most vulnerable and are rapidly depleted. Granulosa cells, active cells that nurture the growing follicles, may also be damaged, leading to death of the oocyte within that follicle.70 Primordial follicle apoptosis,71,72 ovarian atrophy, and cortical fibrosis have also been observed.73 It may seem paradoxical that oocytes resting in a quiescent state as primordial follicles are depleted at an accelerated pace when exposed to chemotherapy. Primordial follicles are recruited out of their inactive state into more vulnerable primary or secondary follicles during chemotherapy, rendering them susceptible to cytotoxic damage.37 Several pathways, including phosphatidylinositol 3-kinase/PTEN/Akt, are involved in activating the primordial follicle, triggering irreversible and unidirectional development to a mature follicle that, under normal circumstances, leads to either ovulation or atresia. In a mouse model, cyclophosphamide resulted in upregulation of the phosphatidylinositol 3-kinase/PTEN/Akt pathway, triggering apoptosis of maturing follicles.74 The concomitant reduction in inhibitory factors normally released by maturing follicles caused increased activation of primordial follicles, with a resultant depletion of this pool.75
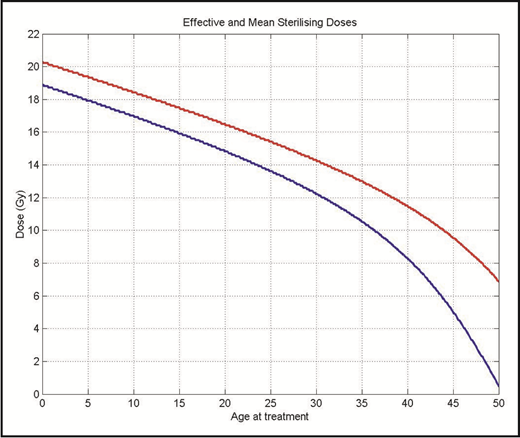
Radiation is directly toxic to oocytes; the dose at which 50% of oocytes die is <2 Gy.76 The age at which permanent ovarian failure occurs depends on the patient’s age at the time of treatment as well as the dose of radiation. The sterilizing dose at age 10 is 18.4 Gy, but falls to 14.3 Gy at age 30 (Figure 3).76 This explains the observation that permanent ovarian failure in women receiving MA total body irradiation is nearly universal. After alloHCT, graft-versus-host disease can target the ovaries and cause ovarian failure.77
The effective (red) and mean (blue) sterilizing dose of radiation for female patients by age at treatment. Reprinted from Wallace et al76 with permission from Elsevier.
The effective (red) and mean (blue) sterilizing dose of radiation for female patients by age at treatment. Reprinted from Wallace et al76 with permission from Elsevier.
Novel therapies: men and women
Some novel agents are gonadotoxic. The US Food and Drug Administration updated its label for bevacizumab after a 34% incidence of ovarian failure (compared with 2% in the control arm) occurred in a phase 2 study.78 Crizotinib, a tyrosine kinase inhibitor (TKI) directed at the anaplastic lymphoma kinase protein, is associated with hypogonadism in men.79 Fertility risks associated with other new therapies are unknown.
Fertility preservation options
Men
Although low sperm counts are common in men with cancer at diagnosis, sperm collection for cryopreservation should be attempted with a goal of acquiring 3 samples before initiation of therapy for future use with intrauterine inseminations or in vitro fertilization (IVF); even with oligozoospermia, intracytoplasmic sperm injection (ICSI) is possible.80,81 Alternative methods of sperm sampling, such as penile vibratory stimulation or electroejaculation,82-85 may be effective for adolescents, or when there are ethical or religious objections or psychosocial barriers to masturbation. In men with azoospermia after treatment, testicular sperm extraction may be performed.86-90 Viable sperm are found in up to 50% of testicular sperm extractions91 and may yield successful pregnancies with IVF-ICSI. Sufficient time after cytotoxic therapy must be allowed to permit nonexposed spermatogonial stem cells to develop into spermatozoa.46
Women
To obtain oocytes for either embryo or oocyte cryopreservation, hormonal stimulation (controlled ovarian hyperstimulation, COH) is used to trigger the simultaneous maturation of numerous follicles. Importantly, there are protocols that are menstrual cycle-independent (random start ovarian stimulation97 ) that result in induction of follicles within 10 to 12 days. Via a transvaginal needle approach, mature oocytes are extracted and then cryopreserved or incubated with sperm (either from a partner or a donor) to create embryos, which are then frozen. There are some drawbacks to oocyte retrieval: delaying cancer-directed therapy for almost 2 weeks may be medically risky; physicians may object to exposing patients to high estrogen levels because of the risk of liver toxicity or thrombosis; and an invasive procedure to obtain oocytes may not be feasible resulting from cytopenias, infections, or cardiopulmonary compromise.
The success of these approaches is dependent on the age of the woman at the time of oocyte retrieval, the method of cryopreservation, and any underlying concomitant endocrine or gynecologic conditions. Maternal age influences oocyte quantity and quality: aneuploidy is associated with increasing age. In 2017, US live birth rates from frozen embryo transfer cycles in the general population ranged from 5% (for women >42 years) to 43% (for women <35 years).98 In a European cohort, cancer survivors experienced live birth rates of up to 44%.99 A US study demonstrated similar cumulative pregnancy rates per transfer (37% in women with cancer vs 43% in age-matched controls).100 Pregnancy rates after embryo cryopreservation in women with cancer appear comparable to those undergoing fertility treatments for other indications.
The method of oocyte cryopreservation (slow freeze or vitrification) affects pregnancy rates. Vitrification is the preferred method because oocyte and embryo survival, fertilization, and pregnancy rates are superior.101
Cryopreservation of oocytes (as opposed to embryos) may be preferred by women without partners or those who may have ethical objections to storing embryonic tissue.102 It also offers an option for women who live in countries that have limitations on embryo cryopreservation.7,102-104 Much of the data regarding pregnancies after frozen oocytes are derived from oocyte donors and suggest comparable live birth rates compared with fresh oocytes,105-108 but a meta-analysis indicates pregnancy rates may be about 25% lower using frozen oocytes.109 Data in cancer survivors are limited, largely because of current oocyte utilization rates <20%. Nonetheless, the available data are reassuring: a single-center study of patients who cryopreserved oocytes before cancer treatment (ages 28-40) demonstrated a 44% live birth rate.110
Ovarian tissue cryopreservation is an experimental technique111,112 in which, via laparoscopy, the entire ovary or sections are harvested and frozen. After completion of cancer therapy, these tissues are reimplanted (usually overlaid on native ovary, Fallopian tube, or peritoneum).113-115 This is the only option for prepubertal girls, and it can be performed immediately, without hormonal stimulation. Reimplantation of ovarian tissue can recapitulate normal hormonal function, preserving not just fertility but also a normal hormonal milieu that may yield other health benefits such as improved bone and cardiovascular health and sexual function. This approach has resulted in pregnancy rates of approximately 30% and endocrine recovery in >90% of women.116,117 However, in both mouse models and humans with hematologic malignancies, low levels of cancer are detectable in ovarian tissue and can result in cancer relapse after reimplantation.118-120 Hence, this approach is contraindicated in women with newly diagnosed blood cancers. The safety of ovarian tissue cryopreservation in remission, particularly for women who may have markers for minimal residual disease which permit testing before reimplantation, remains to be determined.121-124 A single case report suggests this may be feasible.125
Intense controversy surrounds the use of hormonal manipulation, such as gonadotropin releasing hormone analogs (GnRHa), for ovarian protection. Results of some randomized studies and meta-analyses show a benefit, mainly in women with breast cancer, limiting their generalizability.8 Data in hematology patients are scant; 1 study in patients with lymphoma indicated that GnRHa are not effective in preventing chemotherapy-induced POF.126 However, small studies suggest that GnRHa may be protective when administered before HCT.127,128
Fertility may be better preserved with reduced intensity conditioning (RIC) as opposed to MA conditioning before alloHCT.129 However, the reader is urged to recognize the optimal therapy for the patient and to counsel patients carefully regarding the choice of conditioning when weighing fertility concerns. At this time, RIC offers only a theoretical benefit regarding FP, whereas high-quality evidence favors an MA approach for some patients, such as those with acute myeloid leukemia.130 Infertility risks can influence patients’ treatment decisions,131 highlighting the essential importance of patient education and shared decision-making when engaging in treatment planning.
The hematologist must collaborate with the patient and the reproductive specialist in weighing the substantial risks and benefits to these approaches (Table 1). Decision aids can be of great assistance132 and have been developed for young women with breast cancer133 as well as others.134,135 Tools that can be adapted for patients with hematologic malignancies are under development (Nancy N. Baxter, St. Michael’s Hospital, e-mail, 25 February 2019).
Overview of fertility preservation methods
| Intervention . | Eligible patients . | Standard of care? . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Embryo cryopreservation | Adult women | Yes | Well-established method with high live birth rates | Hormonal stimulation required (risk of ovarian hyperstimulation, thrombosis) |
| Offers possibility of preimplantation genetic diagnosis | 10-12 d from initiation of ovarian stimulation until oocyte retrieval using random-start protocols | |||
| Requires a male partner or sperm donor | ||||
| Oocyte cryopreservation | Adult women | Yes | Live birth rates likely similar to embryo cryopreservation | Hormonal stimulation required (risk of ovarian hyperstimulation, thrombosis) |
| Does not require a male partner/sperm donor | 10-12 d from initiation of ovarian stimulation until oocyte retrieval | |||
| May be more preferable for patients with ethical concerns or in countries with restrictions on embryonic tissues | ||||
| Ovarian tissue cryopreservation | Adult women | No | Live birth rates may approach those of oocyte/embryo cryopreservation | Requires laparoscopic abdominal surgery to obtain and to reimplant tissue orthotopically |
| Prepubertal girls | Restores normal hormonal milieu as well as fertility | Risk of malignant cells contaminating tissue | ||
| May be performed immediately (no treatment delay) | ||||
| Only option for prepubertal girls | ||||
| Hormonal manipulation (GnRHa) | Adult women | No | May be only option when cancer-directed therapy must be offered emergently | Unproven efficacy; should not be relied on as sole method of fertility preservation if other options are feasible |
| May protect ovarian function to preserve normal hormonal milieu | Patients may experience menopause-like symptoms | |||
| Menstrual suppression | Osteoporosis | |||
| Oocyte in vitro maturation | Adult women | No | Short course or no ovarian stimulation | Limited numbers of live births |
| May be performed immediately (no treatment delay) | ||||
| No risk of malignant contamination | ||||
| Sperm cryopreservation | Adult men | Yes | Generally readily available | Oligospermia is frequent in men with cancer |
| Low to no risk | Stress of cancer diagnosis or religious/personal/ethical concerns may impair ability to masturbate | |||
| Alternative methods to collect sperm (TESE, penile vibratory stimulus, electroejaculation) are possible | TESE requires surgery to obtain tissue | |||
| Testicular tissue cryopreservation | Adult men | Yes | May be used to obtain sperm for cryopreservation if patient unable to provide a sperm sample | Requires surgery to remove testicular tissue |
| Prepubertal boys | No | Only option for prepubertal boys | Requires surgery to remove testicular tissue | |
| Risk of malignant cells contaminating tissue | ||||
| Development of oocytes or sperm from oogonial or spermatogonial stem cells, embryonic, or induced pluripotent stem cells | All patients | No | May become an option for pre-pubertal children | May require surgery to obtain tissue |
| No risk of malignant contamination | Not yet in clinical use/trials |
| Intervention . | Eligible patients . | Standard of care? . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Embryo cryopreservation | Adult women | Yes | Well-established method with high live birth rates | Hormonal stimulation required (risk of ovarian hyperstimulation, thrombosis) |
| Offers possibility of preimplantation genetic diagnosis | 10-12 d from initiation of ovarian stimulation until oocyte retrieval using random-start protocols | |||
| Requires a male partner or sperm donor | ||||
| Oocyte cryopreservation | Adult women | Yes | Live birth rates likely similar to embryo cryopreservation | Hormonal stimulation required (risk of ovarian hyperstimulation, thrombosis) |
| Does not require a male partner/sperm donor | 10-12 d from initiation of ovarian stimulation until oocyte retrieval | |||
| May be more preferable for patients with ethical concerns or in countries with restrictions on embryonic tissues | ||||
| Ovarian tissue cryopreservation | Adult women | No | Live birth rates may approach those of oocyte/embryo cryopreservation | Requires laparoscopic abdominal surgery to obtain and to reimplant tissue orthotopically |
| Prepubertal girls | Restores normal hormonal milieu as well as fertility | Risk of malignant cells contaminating tissue | ||
| May be performed immediately (no treatment delay) | ||||
| Only option for prepubertal girls | ||||
| Hormonal manipulation (GnRHa) | Adult women | No | May be only option when cancer-directed therapy must be offered emergently | Unproven efficacy; should not be relied on as sole method of fertility preservation if other options are feasible |
| May protect ovarian function to preserve normal hormonal milieu | Patients may experience menopause-like symptoms | |||
| Menstrual suppression | Osteoporosis | |||
| Oocyte in vitro maturation | Adult women | No | Short course or no ovarian stimulation | Limited numbers of live births |
| May be performed immediately (no treatment delay) | ||||
| No risk of malignant contamination | ||||
| Sperm cryopreservation | Adult men | Yes | Generally readily available | Oligospermia is frequent in men with cancer |
| Low to no risk | Stress of cancer diagnosis or religious/personal/ethical concerns may impair ability to masturbate | |||
| Alternative methods to collect sperm (TESE, penile vibratory stimulus, electroejaculation) are possible | TESE requires surgery to obtain tissue | |||
| Testicular tissue cryopreservation | Adult men | Yes | May be used to obtain sperm for cryopreservation if patient unable to provide a sperm sample | Requires surgery to remove testicular tissue |
| Prepubertal boys | No | Only option for prepubertal boys | Requires surgery to remove testicular tissue | |
| Risk of malignant cells contaminating tissue | ||||
| Development of oocytes or sperm from oogonial or spermatogonial stem cells, embryonic, or induced pluripotent stem cells | All patients | No | May become an option for pre-pubertal children | May require surgery to obtain tissue |
| No risk of malignant contamination | Not yet in clinical use/trials |
TESE, testicular sperm extraction.
Women with hematologic malignancies are a special population who face unique challenges in FP
Patients with blood cancers are often acutely ill at presentation. A large mediastinal mass may interfere with the patient’s ability to lay flat for oocyte retrieval or may threaten cardiopulmonary compromise. Hyperleukocytosis, thrombocytopenia, disseminated intravascular coagulation, or infection may not permit an invasive procedure or a delay in therapy. Fertility risks and options should be discussed with these patients. The hematologist’s primary responsibility is to the urgent care of the patient, and often FP must be a secondary concern. In acutely ill patients who must begin cancer-directed therapy straightaway, the only possible intervention may be hormonal, such as GnRHa.136 Menstrual suppression, a side effect of GnRHa, can be beneficial because profound thrombocytopenia may result in menorrhagia. Despite (or perhaps because of) their limited options, women with blood cancers must understand the risks of infertility as early as possible, and ideally before the initiation of cancer-directed therapy, because a strong body of literature indicates that counseling, even without subsequent utilization of a FP intervention, improves quality of life and reduces distress in survivors.137,138
Fortunately, the regimens that are commonly used for the initial treatment of acute leukemia and HL do not usually cause immediate and permanent ovarian failure (<20%).139 High-intensity HL regimens such as bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procarbazine, and prednisone (BEACOPP) and alkylator-based regimens such as cyclophosphamide, doxorubicin, oncovin, and prednisone (CHOP) for non-HL are associated with greater fertility risks, and patients who do not have the time or the ability to undergo oocyte retrieval should be counseled accordingly.140,141 It is especially important to refer all women to reproductive specialists for counseling and possible FP procedures after the completion of therapy because many women will experience POF, even if they resume normal menses after therapy. Also, salvage therapy for relapse includes even more intensive chemotherapy followed almost uniformly by HCT, with its attendant substantial risk of infertility. Therefore, for many women in remission, referral for possible oocyte retrieval is of great importance as this may be their only opportunity to preserve fertility options. AMH levels56 and oocyte yields for these women are lower but sufficient, and healthy pregnancies have been reported,142-145 suggesting that this is safe and feasible.
Women with bone marrow failure syndromes, including aplastic anemia, myelodysplastic syndrome, and GATA2 haploinsufficiency, all of whom may anticipate receiving high-dose therapy including alloHCT, have successfully undergone oocyte retrieval, with a low risk of complications.146,147
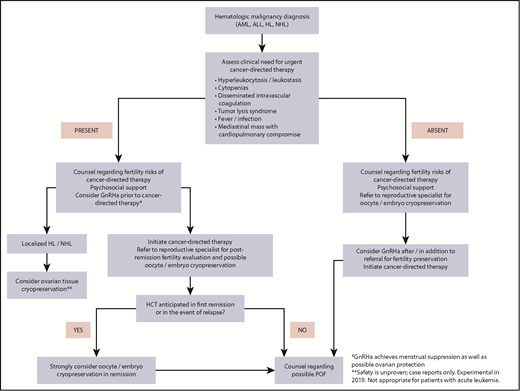
A suggested comprehensive approach to FP in women with hematologic malignancies is outlined in Figure 4.
Fertility preservation approach for women (postpuberty) with newly diagnosed hematologic malignancy.
Fertility preservation approach for women (postpuberty) with newly diagnosed hematologic malignancy.
CML
About 25% of patients with CML are younger than age 50. Most patients present in chronic phase, and lifelong TKI therapy is generally necessary. Men taking imatinib likely can safely father a pregnancy; data for other TKIs are minimal.148,149 TKIs are US Food and Drug Administration pregnancy category D drugs (“positive evidence of human fetal risk”). Women with CML in chronic phase who desire pregnancy must consider several options. Retrieving oocytes before or during TKI therapy allows the greatest flexibility: if a complete molecular remission is achieved and maintained, discontinuation of therapy may be possible150 and a pregnancy could then be pursued in relatively short order. Alternatively, if an optimal response is not obtained, or the patient does not wish to discontinue TKI, her oocytes could be fertilized and a gestational carrier may be used, a complex decision that partly depends on state151 and international laws.152
Some women with CML may elect to stop TKI without meeting “treatment-free remission” criteria for TKI discontinuation153 to permit conception and pregnancy. We offer the opinion that candidates for this approach are women who: express a typical bcr/abl transcript monitored by standard quantitative polymerase chain reaction testing, achieve at least a major molecular response (<0.1%), undergo quantitative polymerase chain reaction bcr/abl testing monthly,153 and be amenable to, and appropriate candidates for, interferon154 if a 1-log rise in bcr/abl occurs.155 Women diagnosed with CML during pregnancy may be observed or managed with interferon.149 Reproductive counseling and options for women with CML must be individualized, and formal guidelines are needed.156,157
Expanding indications for HCT mandate that all hematologists are familiar with FP
Although nonmalignant diseases represent a fraction of transplants performed,158 these numbers are increasing. Autologous HCT has shown promise in several autoimmune diseases,159-161 particularly multiple sclerosis162,163 and systemic sclerosis, for which HCT was recently deemed a standard of care for patients with severe disease.164,165 Compromised fertility may be less frequent in these patients,166,167 possibly because they are less likely to have significant prior exposure to gonadotoxic therapies, but data are very limited.
Reproductive considerations for patients with SCD are complex168 because they may have reduced fertility at baseline. In women, this may be due to inflammation or vaso-occlusion with resultant ovarian ischemia.169 Hypogonadism occurs in up to 24% of men with SCD,170,171 with evidence for both primary and secondary testicular failure. Putative causes include pituitary or testicular infarctions due to vasoocclusion, and iron overload.172 Oligo- and azoospermia are common in men with SCD,173 and hydroxyurea can further lower sperm counts, sometimes irreversibly,174-176 leading some investigators to advocate for sperm cryopreservation before initiating hydroxyurea. Transfusion may improve hormonal profiles and sperm counts177 and could be considered before sperm cryopreservation.
AlloHCT may cure SCD178-180 and is associated with lower health care costs over time and improved health-related quality of life.181,182 Very little is known about fertility risks after HCT for SCD; this is a key question identified in a pediatric consortium position statement.183 MA HCT in children causes high rates of infertility and POF in female patients; male patients may demonstrate either hypergonadotropic or hypogonadotropic hypogonadism, suggesting that some boys may not have achieved puberty, which is often delayed in SCD.180,184,185 A small study of children receiving RIC alloHCT for SCD demonstrates relatively preserved gonadal function.186 Some adults with SCD feel that the possibility of infertility after alloHCT is an unacceptable risk.187 Further studies to understand patient preferences may help guide providers in counseling patients with SCD who are considering transplant.
FP for women with SCD may be risky. Ovarian hyperstimulation syndrome occurs in 1% to 5% of all COH cycles and may cause effusions, renal failure, abdominal pain, and thromboembolism.188 One small series described a high rate of ovarian hyperstimulation syndrome among women with SCD undergoing oocyte retrieval,189 while a series of younger patients suggested fewer complications.190 Ovarian tissue cryopreservation is feasible; the first live birth associated with this technique was reported in a prepubertal girl receiving alloHCT for SCD.191
Education and multidisciplinary programs are essential to facilitate access to reproductive services
Barriers to FP include factors related to providers (lack of comfort with or knowledge about fertility, insufficient time to counsel patients); patients (lack of awareness of risks to fertility or of FP options, concern that their physician may not feel it is appropriate, ethical concerns, parental concerns); and the health care system (cost and insurance coverage; access to reproductive health care).7,28,194-197
American Society for Clinical Oncology guidelines recommend empowering all providers to discuss these issues.8 Direct outreach to patients can improve engagement in FP: providing educational brochures and a facilitated reproductive specialist referral line resulted in a ninefold increase in patient referrals,198 and expedited urology access increased sperm banking sixfold.199
Essentials of oncofertility program building and key resources are outlined in Tables 2 and 3.5,200-202 Fertile Hope has designated Centers of Excellence203 that embody these components.202
Components of a comprehensive oncofertility program
| Institutional commitment . | Policy articulating commitment to fertility needs of patients . |
|---|---|
| Provider education/reminders/empowerment | Grand rounds/conferences |
| Local expert or “champion” to build and maintain program, seek and manage resources | |
| Links to online resources and guidelines (ASCO, NCCN, ESMO, ASRM) (Table 3) | |
| Electronic medical record reminders | |
| Inclusion of fertility risks in consent forms | |
| Shared responsibility by entire team for patient education about fertility | |
| Patient education/support | Discussion of fertility risks before initiation of cancer-directed therapy |
| Brochures/printed materials readily available | |
| Links to online resources (Table 3) | |
| Psychosocial support (therapist, social worker) | |
| Facilitated access to reproductive services | Patient navigator (nurse, social worker) |
| Financial/insurance navigation and/or support | |
| “Hotline”/expedited referral to reproductive specialists who can offer timely (48-72 h) appointments | |
| Build relationships with reproductive consultants who are familiar with all available fertility preservation techniques | |
| Cross-disciplinary collaborations to build relationships |
| Institutional commitment . | Policy articulating commitment to fertility needs of patients . |
|---|---|
| Provider education/reminders/empowerment | Grand rounds/conferences |
| Local expert or “champion” to build and maintain program, seek and manage resources | |
| Links to online resources and guidelines (ASCO, NCCN, ESMO, ASRM) (Table 3) | |
| Electronic medical record reminders | |
| Inclusion of fertility risks in consent forms | |
| Shared responsibility by entire team for patient education about fertility | |
| Patient education/support | Discussion of fertility risks before initiation of cancer-directed therapy |
| Brochures/printed materials readily available | |
| Links to online resources (Table 3) | |
| Psychosocial support (therapist, social worker) | |
| Facilitated access to reproductive services | Patient navigator (nurse, social worker) |
| Financial/insurance navigation and/or support | |
| “Hotline”/expedited referral to reproductive specialists who can offer timely (48-72 h) appointments | |
| Build relationships with reproductive consultants who are familiar with all available fertility preservation techniques | |
| Cross-disciplinary collaborations to build relationships |
ASCO, American Society for Clinical Oncology; ASRM, American Society for Reproductive Medicine; ESMO, European Society for Medical Oncology; NCCN, National Comprehensive Cancer Network.
Oncofertility resources and guidelines for patients and providers
In the United States, as of 2019, only 6 states mandate insurance coverage for patients with iatrogenic infertility (Connecticut, Delaware, Illinois, Maryland, New York, and Rhode Island).203 This coverage is generally limited to standard methods, and may have a cap or a substantial copayment.204 Similar to other complications of cancer treatments, such as breast reconstruction, coverage should be mandatory for iatrogenic infertility.205-207 American cancer survivors describe significant financial burden and stress related to paying for their FP procedures, using more than 20 different ways to cover their costs.208 Adolescents and young adults with cancer are particularly vulnerable to financial toxicity209 ; the substantial cost of FP may act synergistically to increase distress and may impact lifelong financial health and decisions about family-building.
In a recent study of European nations, 14 of 27 responding countries indicated that oocyte/embryo cryopreservation is covered by law.210 Information is scarce regarding FP in low- and middle-income countries, but 1 study demonstrated that barriers are similar to those in higher resourced settings.211 Cultural and legal challenges in some parts of the globe also represent significant hurdles.152
Patients who identify as sexual and gender minorities (SGM) have substantial unmet health care needs.212,213 A qualitative study suggests that lesbian, gay, bisexual, transgender, queer/questioning survivors may be more open to nontraditional forms of family-building and less engaged by existing FP educational materials.212 FP interventions for all SGM individuals contemplating hormonal or surgical treatments are recommended,103,214,215 but uptake of these treatments is low.216 FP for SGM people with cancer is an entirely unexplored field.
Future directions
There are several techniques in development that may open new avenues for FP. Immature oocytes may be retrieved and matured in vitro with either no or minimal ovarian stimulation.217-219 Oocytes and sperm may be developed from immature stem cells, including oogonial stem cells, spermatogonial stem cells,220 gonadal tissue culture,221 and embryonic or induced pluripotent stem cells.222-225 Use of protective agents, such as mTOR inhibitors,226 during chemotherapy may decrease follicular atresia during treatment. Intraovarian artery infusion of granulocyte colony-stimulating factor mobilized HSC may improve ovarian reserve as measured by AMH or AFC and has resulted in a handful of pregnancies in women who were poor responders to COH227,228 ; further studies in cancer patients are needed.
Improving the precision of risk stratification for POF is paramount. AMH, follicle-stimulating hormone, and AFC have all been proposed as biomarkers of ovarian function. Although AMH may predict time to menopause and chemotherapy-induced amenorrhea,229,230 its ability to predict fertility is limited.231 Prediction models must account for age at treatment, prior treatments, prior parity, concomitant medical conditions, and newer putative markers of ovarian reserve.232 Further research is needed to personalize treatment and surveillance plans.
Conclusion
Informing patients facing cancer or HCT about their reproductive risks and options can reduce distress and improve quality of life. The unique needs of patients with hematologic malignancies require a nuanced approach to FP. We must engage in counseling and shared decision-making before initiation of gonadotoxic therapy. Referral to reproductive specialists after completion of therapy is strongly suggested, especially if the provider and the patient agree that pretreatment intervention is not safe or feasible. Advancing technology, improving patient and provider education, facilitating access to reproductive care, and advocating to legislative bodies to support state or nationally mandated coverage is essential to optimize our patients’ reproductive futures.
Authorship
Contribution: A.W.L. and S.S. wrote, reviewed, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alison Wakoff Loren, Division of Hematology/Oncology, Department of Medicine, Perelman School of Medicine, 6 S Pavilion, University of Pennsylvania, Philadelphia, PA 19104; e-mail: alison.loren@uphs.upenn.edu.