Key Points
Frontline bendamustine and rituximab in advanced stage FL has marked efficacy in this population-based analysis.
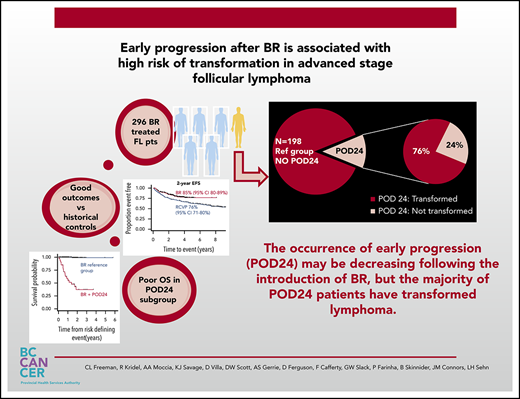
Early progression within 24 months is associated with poor outcome after BR; however, the majority of patients have transformed lymphoma.
Abstract
Despite widespread use of bendamustine and rituximab (BR) as frontline therapy for advanced-stage follicular lymphoma (FL), little is known about the risk of early progression or incidence of histological transformation. We performed a retrospective analysis of a population-based cohort of 296 patients with advanced-stage FL treated with frontline BR and maintenance rituximab. As previously demonstrated, outcomes with this regimen are excellent, with 2-year event-free survival estimated at 85% (95% confidence interval [95% CI], 80-89) and 2-year overall survival 92% (95% CI, 88-95). Progression of disease within 24 months (POD24) occurred in 13% of patients and was associated with a significantly inferior outcome with 2-year overall survival of 38% (95% CI, 20-55). The only significant risk factor for POD24 at baseline was elevated lactate dehydrogenase (P < .001). Importantly, the majority of POD24 patients (76%) had transformed disease. Compared with a historical cohort treated with rituximab, cyclophosphamide, vincristine, and prednisone, event-free survival has improved and the risk of POD24 has decreased, but a higher proportion of patients with POD24 harbor transformation. The overall incidence of transformation appears unchanged. The presence of occult or early transformation is the main driver of POD24 in FL patients treated with frontline BR. Identification of biomarkers and improved management strategies for transformation will be crucial to improving outcomes.
Introduction
Bendamustine and rituximab (BR) has become a preferred regimen for frontline treatment of patients with advanced-stage follicular lymphoma (FL) since randomized trials demonstrated impressive efficacy and a favorable toxicity profile.1,2 The outcomes of FL patients that progress early, within 24 months of diagnosis or treatment initiation (POD24), are consistently inferior in large retrospective analyses.3-7 The incidence of POD24 and transformation after BR has not been widely reported.
BR followed by 2 years of maintenance rituximab (MR) has been the recommended frontline therapy for advanced stage, symptomatic FL in British Columbia (BC) since 2013. In this population-based analysis, we evaluated outcomes, the incidence of POD24, and histological transformation and compared them with a historical cohort of patients treated with frontline rituximab, cyclophosphamide, vincristine, and prednisone (RCVP) and MR, the previous standard induction in BC.
Methods
This retrospective study was approved by the University of British Columbia Cancer Research Ethics Board. The BC Cancer Lymphoid Cancer Database was used to identify patients at least 18 years of age with histologically confirmed FL grades 1-3A with symptomatic advanced stage disease (Ann Arbor stages III-IV or I/II if bulky or not amenable to radiation) who received BR as their first systemic therapy before 1 April 2018. Diagnostic biopsies were centrally reviewed at the time of diagnosis or treatment initiation and categorized according to the World Health Organization classification.8
Patients were excluded if they were HIV+ or had known coexistent high-grade lymphoma. Observation before systemic therapy was permitted, but patients were excluded if they received radiation or rituximab monotherapy before BR. Clinical and follow-up information was obtained from the Lymphoid Cancer Database, BC Cancer, and hospital records, or individual physicians’ records. Outcomes were compared with a historical cohort of patients treated with frontline RCVP (2005-2013). All those included were eligible to receive 2 years of MR, which is standard for responding patients after induction in BC.
It was not routine practice in BC for FL patients to undergo a staging positron emission tomography (PET) scan during this period, nor was PET scanning performed for response evaluation or surveillance during follow-up. Our monitoring policy follows accepted practice in that patients undergo response assessment posttreatment (generally by computed tomography scan) and then are seen at periodic intervals to monitor for treatment complications and assess for progression. During maintenance therapy, patients are seen before each scheduled dose. Following completion of therapy, patients are seen and assessed according to physician discretion. In general, patients are seen every 4 to 6 months, and imaged when clinically indicated. When necessary, computed tomography scanning is the routinely used imaging modality, although a PET scan is frequently performed if transformation is clinically suspected.
Event-free survival (EFS), overall survival (OS), and time to transformation were calculated from the date of initiation of systemic therapy. The Kaplan-Meier method was used to estimate survival probability and generate survival curves.9 The presence of transformation was based on biopsy confirmation when feasible, with the finding of high-grade histology and classified in accordance with the World Health Organization.8 In the minority of cases where biopsy was not feasible, a clinical diagnosis of transformation was based on previously established criteria, with the presence of at least 1 of the following: sudden rise in lactate dehydrogenase (LDH) to twice upper limit of normal; rapid discordant localized nodal growth detected clinically or by imaging studies; new involvement of unusual extranodal sites (eg, liver, bone, muscle, brain); new B-symptoms; or the development of hypercalcemia.10,11
Early progression (POD24) was defined as progression or relapse, death from lymphoma, or treatment toxicity within 24 months of initiation of systemic therapy. Similar to methodology previously published, patients were grouped into 2 categories: those with an event defined as POD24 or those who reached the 24-month landmark without a POD24 defining event (reference group).3 Patients lost to follow-up or those that died of causes unrelated to lymphoma within 24 months were not included in the POD24 group. Univariate associations between baseline characteristics and POD24 or transformation were tested using the Pearson χ2 test.
Results and discussion
In total, 296 patients meeting eligibility criteria were identified. Median age was 61 years (range, 24-86), 83% had stage III/IV disease and 73% had an intermediate or high-risk Follicular Lymphoma International Prognostic Index (FLIPI) score. Baseline characteristics of the BR population and the historical RCVP-treated patients (N = 347) are outlined in Table 1. Characteristics were largely similar between the cohorts, although fewer BR patients were initially observed because of a policy change in BC enabling the use of rituximab monotherapy in asymptomatic patients, and there were slightly more patients with low FLIPI score in the BR cohort. Nonetheless, a FLIPI-adjusted Cox model correcting for clinical differences demonstrated no effect on the estimated effect of frontline BR (data not shown).
Baseline characteristics of BR-treated cohort compared with an historical RCVP-treated cohort
| Characteristic . | BR (N = 296), n . | % . | RCVP (N = 347), n . | % . | P . |
|---|---|---|---|---|---|
| Age >60 y | 153 | 52 | 189 | 54 | .5 |
| Male | 163 | 55 | 195 | 56 | .8 |
| Ann Arbor stage III/IV | 245 | 83 | 305 | 88 | .02 |
| Elevated LDH | 67 | 23 | 73 | 23 | .9 |
| Bone marrow involved | 136 | 46 | 178 | 51 | .2 |
| B-symptoms | 68 | 23 | 78 | 22 | .4 |
| Low hemoglobin <120 g/L | 47 | 16 | 61 | 19 | .3 |
| FLIPI (missing) | 6 | 2 | 52 | 15 | |
| Low (0-1) | 74 | 25 | 43 | 12 | .002 |
| Intermediate (2) | 106 | 36 | 107 | 31 | |
| High (3-5) | 110 | 37 | 145 | 42 | |
| FL grade 1/2 | 254 | 87 | 295 | 85 | .5 |
| FL grade 3A | 39 | 13 | 52 | 15 | |
| Initial observation | 34 | 11 | 87 | 25 | <.001 |
| Maintenance rituximab | 243 | 84 | 284 | 82 | .4 |
| Characteristic . | BR (N = 296), n . | % . | RCVP (N = 347), n . | % . | P . |
|---|---|---|---|---|---|
| Age >60 y | 153 | 52 | 189 | 54 | .5 |
| Male | 163 | 55 | 195 | 56 | .8 |
| Ann Arbor stage III/IV | 245 | 83 | 305 | 88 | .02 |
| Elevated LDH | 67 | 23 | 73 | 23 | .9 |
| Bone marrow involved | 136 | 46 | 178 | 51 | .2 |
| B-symptoms | 68 | 23 | 78 | 22 | .4 |
| Low hemoglobin <120 g/L | 47 | 16 | 61 | 19 | .3 |
| FLIPI (missing) | 6 | 2 | 52 | 15 | |
| Low (0-1) | 74 | 25 | 43 | 12 | .002 |
| Intermediate (2) | 106 | 36 | 107 | 31 | |
| High (3-5) | 110 | 37 | 145 | 42 | |
| FL grade 1/2 | 254 | 87 | 295 | 85 | .5 |
| FL grade 3A | 39 | 13 | 52 | 15 | |
| Initial observation | 34 | 11 | 87 | 25 | <.001 |
| Maintenance rituximab | 243 | 84 | 284 | 82 | .4 |
With a median follow-up of 3.1 years (range, 0.2-8) for BR-treated patients, the 2-year EFS is 85% (95 confidence interval [95% CI], 80-89] and 2-year OS is 92% (95% CI, 88-95) (supplemental Figure 1A-B , available on the Blood Web site). Twenty-nine (10%) BR-treated patients had evidence of transformation, the majority (69%) being histologically confirmed. Median time to transformation was 8.4 months (range, 2.7-48 months). Transformation represented 53% of all progression/relapse events observed. Posttransformation outcome was poor, with 2-year OS 40% (95% CI, 20-59). Only elevated LDH at baseline was associated with an increased risk of transformation (P < .001). Grade 3A FL was not associated with increased transformation risk.
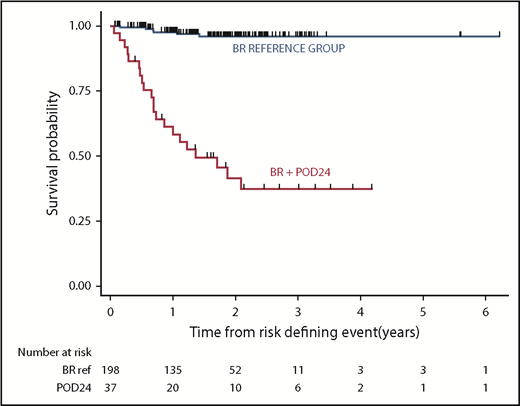
POD24 occurred in 37 (13%) of BR-treated patients, with an elevated LDH (P < .001) at baseline being the only identifiable risk factor. The majority, 28/37 (76%), had transformed disease. Grade 3A was not associated with increased risk of POD24. Outcome in patients experiencing POD24 was poor, with postprogression 2-year OS of 38% (95% CI, 20-55) (Figure 1).
OS from a risk-defining event occurring after initiation of therapy with bendamustine and rituximab in patients with advanced stage FL treated in BC. Outcomes in patients experiencing early progression (POD24) were poor, with 2 year postprogression survival estimated at 38% (95% CI 20-55%), which is markedly inferior to that observed in the reference group.
OS from a risk-defining event occurring after initiation of therapy with bendamustine and rituximab in patients with advanced stage FL treated in BC. Outcomes in patients experiencing early progression (POD24) were poor, with 2 year postprogression survival estimated at 38% (95% CI 20-55%), which is markedly inferior to that observed in the reference group.
Compared with the historical cohort of patients treated with RCVP (with median follow-up 8.5 years), the introduction of BR has improved EFS (2-year EFS, 85% vs 76%, P = .001) and decreased the incidence of POD24 (13% vs 23%, P = .001). No significant difference in OS is demonstrated (supplemental Figure 1A-B). Although the probability of developing transformation appears similar with the available follow-up (supplemental Figure 2), among patients with POD24, a higher proportion of patients treated with BR have evidence of transformation compared with RCVP (76% vs 40%).
Our population-based analysis demonstrates that in the absence of transformation or POD24, patients with advanced stage FL have excellent outcomes following frontline BR and maintenance rituximab. The incidence of early progression with FL-only histology is uncommon following BR because it appears that the majority of POD24 patients in this population have transformed lymphoma. Our findings reflect real-world outcomes following 1 of the most commonly used frontline therapies for FL and confirm those seen in recent clinical trials that identified a lower incidence of POD24 in patients treated with BR compared with RCVP (16.1% vs 26.9%), highlighted the poor outcomes for those with POD24, and identified that the baseline factor most strongly associated with risk of POD24 was serum LDH.5,12
Our study is unique in reporting real-world outcomes after upfront-BR for patients with advanced-stage FL and should largely reflect an unselected patient population. It is striking that the large majority of patients (76%) with POD24 following treatment with BR in our population-based cohort had evidence of transformed lymphoma. This is notably higher than reported in the GALLIUM trial, which reported a transformation rate of only 19.4% (19/98) in R-chemo-treated POD24 patients.5 The GALLIUM study, similar to all randomized controlled trials, is likely composed of a lower risk patient population, which may have influenced the differences observed. Importantly, details on transformation rate are notoriously difficult to capture within clinical trials, possibly because of variable practices on repeat biopsy in individual centers, and underreporting by investigators once progression is documented. In addition, treatment in the GALLIUM trial was heterogeneous, and the risk of transformation within each chemotherapy arm has not been reported. Although PET scans at staging were not routinely performed in our cohort during this period, staging PET scans performed in a subset of patients in the GALLIUM trial failed to identify risk of subsequent transformation.13
POD24 certainly constitutes an unmet medical need in FL and studies investigating novel therapies and targeted approaches are under way. However, our findings indicate that improving outcomes in this cohort will require approaches that enable better detection and improved treatment of transformed disease.
Patients who experience transformation can have evidence of the aggressive clone at the time of diagnosis, documented by analysis of circulating tumor DNA, or deep sequencing of the diagnostic tissue biopsy.14,15 However, comprehensive genetic, biological, or radiological tools applied at baseline have not proven sufficiently sensitive, and these tools may not retain their predictive capacity in patients treated with BR.13,16,17 Risk of POD24 and transformation may be altered with improved therapies. There are some data to suggest that consolidative autologous transplantation after POD24 may have utility, although this may reflect its effect on occult transformation.18 Based on our findings, clinicians should be aware of the high risk of transformation in FL with POD24 after frontline BR, pursue biopsy confirmation wherever possible and consider when selecting next lines of therapy. Future research should focus on identification of key biomarkers and improved management strategies for those with transformed lymphoma.
These data were presented in part at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.L.F. was involved in data collection and designed and performed the analysis; R.K., A.A.M., D.F., and F.C. were involved in data collection; L.H.S. also was involved in data analysis; and all authors were involved in writing the manuscript.
Conflict-of-interest disclosure: C.L.F. receives honoraria: Seattle Genetics, Janssen, Amgen, Celgene, AbbVie. D.W.S. receives patents and royalties as a named inventor on a patent licensed to NanoString Technologies; research funding from NanoString Technologies, Janssen, and Roche; consultancy and travel funds: Celgene. J.M.C. receives research funding from Merck, Janssen, Bristol Myers-Squibb, Cephalon, Bayer Healthcare, Seattle Genetics, Genentech, Lilly, F Hoffmann-La Roche, Roche Canada, Takeda, Amgen; patents and royalties as a named inventor on a patent licensed to NanoString Technologies; and honoraria from Seattle Genetics and Takeda. L.H.S. provides consultancy and receives honoraria from Karyopharm, AbbVie, Seattle Genetics, Amgen, Celgene, Roche/Genentech, Morphosys, TG Therapeutics, Merck, Lundbeck, and Janssen. A.S.G. receives research funding to her institution from Roche, Lundbeck, Janssen, and Accerta; honoraria from Roche, Janssen, and AbbVie; and serves on an advisory board for Janssen, AbbVie, and Celgene. K.J.S. receives institutional funding from Roche Villa, Janssen, Roche, Celgene, Lundbeck, Seattle Genetics, AstraZeneca, Gilead, and AbbVie; and D.R.V. receives honoraria from Janssen, Roche, Lundbeck, Celgene, Seattle Genetics, AbbVie, AstraZeneca, Gilead, and Nanostring. R.K. receives research funding from Gilead and travel support from Roche.
Correspondence: Ciara Freeman, Department of Medical Oncology, BC Cancer, 600 W 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: ciara.freeman@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal