Background: The minimal or measurable residual disease (MRD) status post induction-consolidation is widely accepted as major surrogate endpoint that correlates with outcome in adult patients with newly diagnosed acute lymphoblastic leukemia (ALL). However, the optimal time-points of MRD assessment at complete remission (CR) or later during consolidation and its impact on outcome remain unclear.
Methods: We performed a retrospective chart review of 419 newly diagnosed adult patients with Philadelphia negative B-cell ALL who received treatment at the M.D. Anderson Cancer Center between 01/2000 and 01/2015 (Figure 1). Overall, 394 of 419 (94%) patients achieved CR. Two hundred and fifteen patients (51%) had available MRD assessment (by multicolor flow cytometry) at CR (1st time-point: median 24 days) and first post-CR MRD assessment (2nd time-point: median 3.6 months), and constitute the current studied cohort. MRD responses were stratified as MRD negative (undetectable), low-positive (≤0.1%), and high-positive (>0.1%).
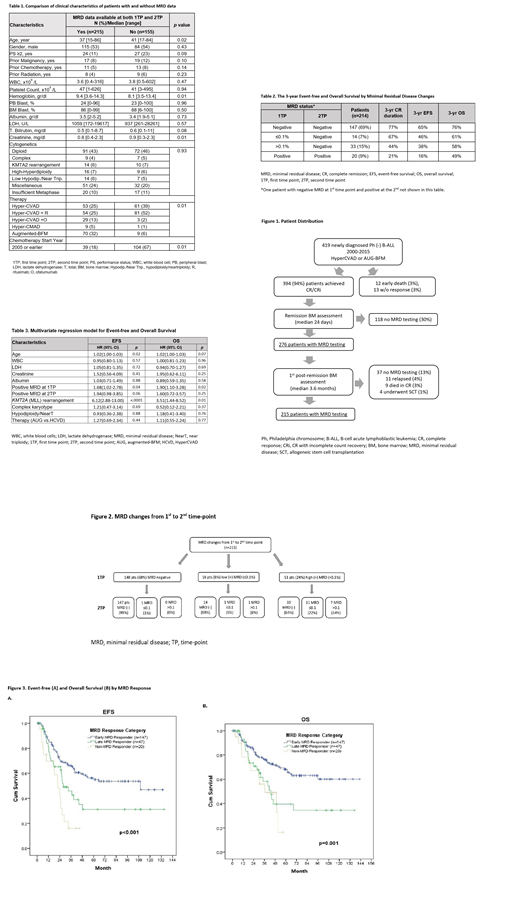
Results: The median age for the study cohort (n=215) was 37 years (range 15-86), and 17% (n=37) had unfavorable cytogenetic profile, such as complex karyotype (n=9), KMT2A (MLL) rearrangement (n=14), and low hypodiploidy/near triploidy (n=14) (Table 1).
At first time-point (1TP), 148 patients (68%) were MRD negative and 67 (32%) were positive (Figure 2). Of the 148 patients with negative MRD at 1TP, 147 (99%) maintained it through second time-point (2TP). Fourteen of 16 (88%) patients with low-positive MRD and 33 of 51 (64%) with high-positive MRD at 1TP became MRD negative at 2TP. Patients who were MRD negative at both time-points, early MRD responders, had the 3-year event-free survival (EFS) and overall survival (OS) rates of 65% and 76%, respectively (Table 2). Patients with improved MRD status from positive to negative, late MRD responders, had lower 3-year EFS and OS rates, 42% and 58%, respectively (Figure 3, p<0.01). The 3-year EFS and OS rates were the lowest (16% and 49%, respectively) in patients with persistent MRD at both time points (p<0.01). Multivariate analysis showed that older age, KMT2A (MLL) rearrangement, and MRD positivity at 1TP only correlated with worse survival (Table 3).
Conclusion: The early achievement of MRD negative CR is a strong predictive for survival. Innovative combinations of conventional chemotherapy and targeted agents that induce early MRD eradication may have the potential to improve cure rates in adult ALL.
Kantarjian:Daiichi-Sankyo: Research Funding; Astex: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Cyclacel: Research Funding; Novartis: Research Funding; Ariad: Research Funding; Immunogen: Research Funding; Amgen: Honoraria, Research Funding; Agios: Honoraria, Research Funding; BMS: Research Funding; Takeda: Honoraria; Jazz Pharma: Research Funding; AbbVie: Honoraria, Research Funding. Khoury:Kiromic: Research Funding; Angle: Research Funding; Stemline Therapeutics: Research Funding. Ravandi:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Menarini Ricerche: Research Funding; Macrogenix: Consultancy, Research Funding; Selvita: Research Funding. Short:AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Honoraria. Loghavi:GLG Consultants: Consultancy; AlphaSights: Consultancy; MDACC: Employment. Cortes:BiolineRx: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Biopath Holdings: Consultancy, Honoraria. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Konopleva:Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Wierda:Gilead Sciences: Research Funding; Juno Therapeutics: Research Funding; KITE pharma: Research Funding; Sunesis: Research Funding; Miragen: Research Funding; Oncternal Therapeutics Inc.: Research Funding; Cyclcel: Research Funding; Acerta Pharma Inc: Research Funding; Pharmacyclics LLC: Research Funding; Genentech: Research Funding; AbbVie: Research Funding; GSK/Novartis: Research Funding; Loxo Oncology Inc.: Research Funding; Janssen: Research Funding; Xencor: Research Funding. Jain:Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding. Verstovsek:Pragmatist: Consultancy; Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy. Bose:Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding; Blueprint Medicine Corporation: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Constellation: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; CTI BioPharma: Research Funding. O'Brien:Sunesis: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Regeneron: Research Funding; AbbVie: Consultancy, Honoraria; Acerta: Research Funding; Alexion: Consultancy; Gilead: Consultancy, Research Funding; Eisai: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Amgen: Consultancy; Kite: Research Funding; TG Therapeutics: Consultancy, Research Funding; Vaniam Group LLC: Consultancy; Verastem: Consultancy; Aptose Biosciences, Inc: Consultancy; GlaxoSmithKline: Consultancy; Janssen: Consultancy, Honoraria; Celgene: Consultancy; Astellas: Consultancy. Jabbour:Adaptive: Consultancy, Research Funding; Cyclacel LTD: Research Funding; BMS: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal