Background: Axi-cel is the first, personalized autologous anti-CD19 CAR T cell therapy approved for the treatment of patients with relapsed/refractory large B cell lymphoma (LBCL) with ≥ 2 prior systemic therapies (YESCARTA. Prescribing Information. 2017). With a median follow-up of 27.1 months in ZUMA-1, the overall response rate was 83%, and 39% of the treated patients had ongoing response (Locke et al. Lancet Oncol. 2019). To gain insights into the mechanism of secondary treatment failure post-axi-cel, as well as define alternative targets and product optimization approaches, we analyzed tumor biopsies obtained prior to axi-cel therapy and at relapse.
Methods: Tumor tissue samples from patients in Cohorts 1 and 2 of ZUMA-1 who had responded and subsequently relapsed were assessed in a post-hoc analysis for protein expression of B cell linage markers (CD19, CD20, PAX5, CD79a, and CD22) by multiplex immunohistochemistry (IHC), followed by multiplex immunofluorescence (IF) staining and confocal microscopy in representative cases. Pretreatment tissue samples were available from 96 patients, and 21 were available post-relapse. Paired pretreatment and post-relapse samples were available for 16 patients. CD19 and CD20 H-scores were derived based on proportion and intensity of antigen expression. Scores of 0 - 5 were considered negative, and scores of 6 - 300 were considered positive. CD19 splice variants were assessed by RNA sequencing.
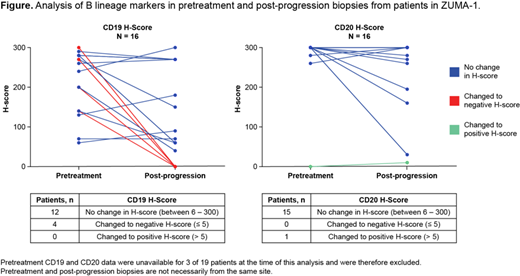
Results: Among all patients with available post-relapse samples, 7/21 (33%) showed loss of CD19 expression. Analysis of the 16 paired pretreatment and post-treatment samples showed loss of CD19 expression in 4 patients (25%) who relapsed post-axi-cel (Figure). Nineteen post-relapse tumor samples were evaluable for other B cell lineage markers and showed preservation of CD20, CD22 and CD79a, and the B cell lineage transcription factor PAX5, even in samples with loss or substantial reduction of CD19 expression. Multiplex IF showed that CD19 and CD20 were expressed on the cell membrane, and analysis uncovered the presence of malignant cells with different relative expression levels of these two antigens within a given biopsy. Interestingly, among the 96 pretreatment tumor samples, IHC analysis demonstrated that CD20 was robustly expressed in nearly all samples, alongside CD19, despite all patients having previously relapsed after receiving rituximab-based regimens. The CD19 and CD20 expression levels in these tumor biopsies obtained pre-axi-cel did not correlate with each other.
RNA sequencing showed alternative splicing of CD19 with loss of exon 2 and/or exons 5/6 in diffuse large B cell lymphoma tumors at baseline and/or relapse, similar to what has been described previously in B cell acute lymphoblastic leukemia (Sotillo et al. Cancer Discov. 2015). In addition, several novel splice junctions have been identified. Data on the correlation between H-scores and CD19 splice forms and clinical outcomes, including response and progression-free survival, will be presented.
Conclusions: In this cohort of patients relapsing after axi-cel, loss of CD19 expression was common by IHC as compared to pretreatment, likely due to alternative splicing and selection of variants devoid of target epitope. Additionally, the data showed that expression of alternate B cell lineage antigens was largely preserved. In particular, CD20 cell surface expression was strong in most tumors despite prior rituximab-based treatments. Altogether, these data point to strategies to improve efficacy of anti-CD19 CAR T cell products through co-targeting or sequential targeting of alternate B cell antigens.
Neelapu:Novartis: Consultancy; Cell Medica: Consultancy; Poseida: Research Funding; Cellectis: Research Funding; Precision Biosciences: Consultancy; BMS: Research Funding; Pfizer: Consultancy; Unum Therapeutics: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Incyte: Consultancy; Celgene: Consultancy, Research Funding; Acerta: Research Funding; Karus: Research Funding; Allogene: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. Rossi:Kite, A Gilead Company: Employment. Jacobson:Celgene: Consultancy, Other: Travel Expenses; Novartis: Consultancy, Honoraria, Other: Travel Expenses; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Pfizer: Consultancy, Research Funding; Humanigen: Consultancy, Other: Travel Expenses; Bayer: Consultancy, Other: Travel Expenses; Precision Biosciences: Consultancy, Other: Travel Expenses. Locke:Novartis: Other: Scientific Advisor; Cellular BioMedicine Group Inc.: Consultancy; Kite: Other: Scientific Advisor. Miklos:AlloGene: Consultancy; Celgene-Juno: Consultancy; Adaptive Biotechnologies: Consultancy, Research Funding; Janssen: Consultancy; Novartis: Consultancy; Kite, A Gilead Company: Consultancy, Research Funding; Becton Dickinson: Consultancy; Miltenyi: Consultancy, Research Funding; Pharmacyclics: Consultancy, Patents & Royalties, Research Funding; Precision Bioscience: Consultancy. Reagan:Kite, A Gilead Company: Consultancy; Curis: Consultancy; Seattle Genetics: Research Funding. Rodig:Kite, a Gilead Company: Research Funding; Bristol Myers Squib: Consultancy, Honoraria, Other: Travel Expenses, Speakers Bureau; Merck: Research Funding; Affirmed: Research Funding. Flinn:F. Hoffmann-La Roche Ltd: Research Funding; TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding; Acerta Pharma, Agios, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Genentech, Gilead Sciences, Incyte, Infinity Pharmaceuticals, Janssen, Karyopharm Therapeutics, Kite Pharma, Novartis, Pharmacyclics, Portola Pharmaceuticals: Research Funding; AbbVie, Seattle Genetics, TG Therapeutics, Verastem: Consultancy; TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding. Milletti:Gilead: Employment, Equity Ownership, Other: Travel Expenses, Patents & Royalties; Roche: Employment, Equity Ownership, Other: Travel Expenses, Patents & Royalties; Kite, a Gilead Company: Employment. Chang:Kite, a Gilead Company: Employment, Equity Ownership. Xue:Kite, a Gilead Company: Employment. Plaks:Gilead: Equity Ownership. Kim:Kite, a Gilead Company: Employment. Bot:Kite, a Gilead Company: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal