Introduction: Paroxysmal nocturnal hemoglobinuria (PNH) is a non-malignant clonal disease of hematopoietic cells with a complex pathophysiology and variable clinical spectrum, characterized by signs and symptoms related to intravascular hemolysis, hypercoagulability, and cytopenias. Metaboloma is the end product of an organism's genetic configuration plus the influence of all factors to which it is exposed. Metabolomic profile is able to provide a more accurate functional measure of a phenotype formed by the result of genomic, transcriptomic and proteomic changes.
Aims: To compare a metabolomic profile in the PNH hemolytic group with healthy controls, and to compare a metabolomic profile before and after the administration of eculizumab in PNH patients.
Methods: To perform metabolomic profile, we used mass spectrometry in 23 patients with hemolytic PNH and in 166 healthy adults, as control group. Twelve PNH patients samples were also collected before and 24 hours after receiving eculizumab. Liquid chromatography with mass spectrometry was performed using the AbsoluteIDQ P180 Biocrates kit (Biocrates, Life Science AG, Innsbruck, Austria): 186 metabolites from 7 different classes were identified and quantified. The data were imported to the analytical site MetaboAnalyst 4.0 and ROCCET: ROC Curve Explorer & Tester for the generation of Univariate and Multivariate Operational Characteristic curves of the Receiver (ROC).
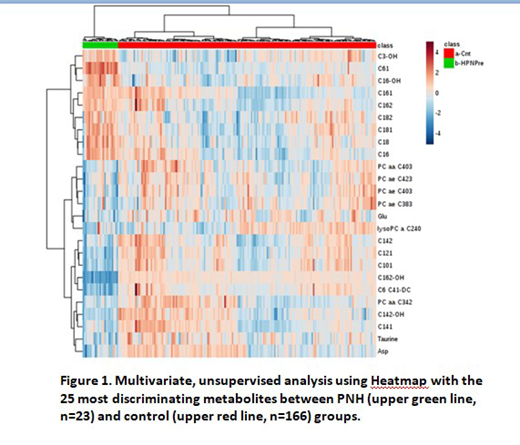
Results: In the patients with hemolytic PNH, of the 186 metabolites analyzed, 92 of them showed significant differences between patients and controls, with positive or negative correlation. The major metabolites increased in the PNH group were long-chain acylcarnitines, while the major metabolites reduced in the PNH group were histidine, taurine, glutamate, glutamine, aspartate and phosphatidylcholines. The C14:1/C16:1 ratio lower than 1.4 was shown to be a reliable marker in patients with PNH, suggesting a disorder in lipid elongation. Besides, PNH patients had a significant increase in C6:1 levels and C14:1/C6 and C4/C6 ratios. After receiving eculizumab, patients reached levels comparable to those of normal subject.
Conclusion: We observed differences in the metabolome of PNH patients as compared to healthy controls, suggesting a unique metabolomic profile of these patients, characterized by an altered acylcarnitine balance, reduction in aminoacids participating in the glycogenesis pathway and an impaired glutaminolysis. Acylcarnitines levels seem to significantly reduce with the use of eculizumab, demonstrating that the use of the antibody has action in reducing hemolysis and possibly in mitochondrial function of these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal