Background: Cytogenetic abnormalities have prognostic implications in acute lymphoblastic leukemia (ALL). High risk cytogenetics include complex karyotype (≥ 5 anomalies), low-hypodiploidy / near-triploidy, 14q32/IGH rearrangements and 11q23/KMT2A rearrangements. The most frequent gene partner involved in KMT2A-rearranged ALL is AFF1 located on chromosome 4q21. ALL with t(4;11)(q21;q23) - KMT2A-AFF1 has a very poor outcome and patients (pts) with this translocation are offered allogeneic stem cell transplantation (HSCT) in first complete remission (CR1). More than 130 gene partners have been described in KMT2A rearrangements. The frequency and prognostic signification of these various gene partners in KMT2A-rearranged ALL is unknown and it is uncertain whether pts harboring these translocations should be offered HSCT in CR1.
Methods: We retrospectively reviewed 1102 pts with newly diagnosed ALL treated at our institution between 1984 and 2019 to identify pts with KMT2A rearrangement. Presence of t(11;v)(q23;v) was assessed by conventional cytogenetics with G-banding and/or by fluorescence in situ hybridization (FISH). Clinical and laboratory data were collected retrospectively. We analyzed the pts characteristics at baseline and evaluated remission rates, overall survival (OS) and relapse-free survival (RFS). The survival curves were estimated using the Kaplan-Meier method and differences between groups were evaluated with the log-rank test. Univariate Cox proportional hazard ratio were used for estimation of hazard ratios (HR). HSCT in CR1 was considered as a time-dependent covariate.
Results: We identified 51 cases (5%) of ALL with KMT2A rearrangement or amplification. Two cases (4%) were cryptic KMT2A rearrangements identified by FISH, but with a normal karyotype. The most common KMT2A rearrangement was t(4;11)(q21;q23) in 42/51 (82%) pts. Four (8%) pts had t(11;19)(q23;p13) in which KMT2A can be partnered with MLLT1 or ELL, 1 (2%) pt had t(9;11)(p21;q23) - KMT2A-MLLT3, 1 (2%) pt had t(11;15)(q32;q26) with unknown gene partner, 1 (2%) pt had hsr(11)(q23) with KMT2A amplification, and 2 (4%) pts had an unknown gene partner (identification by FISH). Sixteen (31%) pts had additional chromosomal abnormalities, with i(7q) (3/51, 6%) and + X (2/51, 4%) the only identified in more than one case.
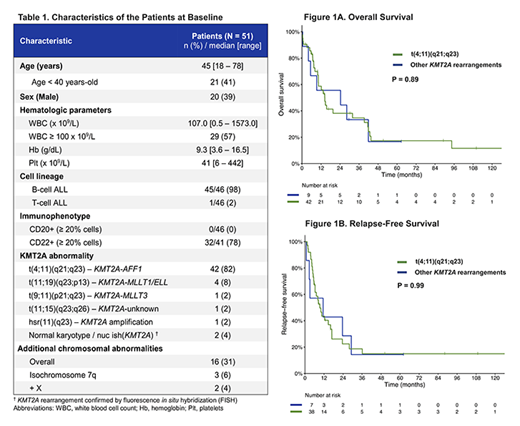
The pts' clinical characteristics are summarized in table 1. All but one were B-cell ALL. The median age at diagnosis was 45 year-old (range, 18 - 78). The median white blood cell count (WBC) at diagnosis was 107.0 x 109/L (range, 0.5 - 1573.0) and 29/51 (57%) pts had hyperleukocytosis with WBC ≥ 100 x 109/L. None of the cases of B-ALL had CD20 expression, but 32/41 (78%) had CD22 expression (median of 59%; range 0 - 99%).
Pts were treated with various regimens, with most pts receiving the Hyper-CVAD regimen (n=35), or one of its variation including addition of anti-CD20 monoclonal antibody (n=2), blinatumomab (n=1), nelarabine for T-cell ALL (n = 1) or dose adjusted regimen with omission of doxorubicin (mini-Hyper-CVD, n = 4). The complete remission rate was 88% (45/51) with 5 (10%) deaths during induction and 1 (2%) refractory disease. The measurable residual disease (MRD) negativity rate was 57% (17/30 evaluable pts). With a median follow-up of 63 months, the median OS and RFS were 14 months (95% confidence interval [CI], 10 - 39] and 10 months (95% CI, 6 - 17), respectively. The 5-year OS and RFS rates were 17% (95% CI, 9 - 35%) and 15% (95% CI, 7 - 33%), respectively. Comparing the 42 pts with t(4;11) and the 9 pts with other KMT2A abnormalities, no difference in OS (HR 0.94, p = 0.89) and RFS (HR 1.00, p = 0.99) were observed (Figure 1A-1B). HSCT in CR1 was associated with a better outcome in terms of OS (HR 0.49, 95% CI 0.24 - 0.99, p = 0.049) and RFS (HR 0.44, 95% CI 0.22 - 0.92). In the 17 (33%) pts who underwent HSCT in CR1 (16 with t(4;11) and 1 with other KMT2A rearrangement), the 5-year OS and RFS rates were 30% (95% CI, 14 - 68%) and 24% (95% CI, 9 - 62%), respectively, versus 10% (95% CI, 3 - 35%) and 9% (95% CI, 3 - 34%), respectively, in the 34 (67%) pts who did not undergo HSCT. The only 2 pts with KMT2A rearrangements other than t(4;11) who remain alive are the only 2 who have undergone HSCT: 1 in CR1 and 1 in CR2.
Conclusion: Patients with KMT2A-rearranged ALL have a poor prognosis, which do not differ based on the gene partner involved. These patients benefit from HSCT in CR1.
Kantarjian:BMS: Research Funding; Astex: Research Funding; Pfizer: Honoraria, Research Funding; Ariad: Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Immunogen: Research Funding; Jazz Pharma: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; Cyclacel: Research Funding; Novartis: Research Funding; Daiichi-Sankyo: Research Funding; Takeda: Honoraria. Khoury:Kiromic: Research Funding; Angle: Research Funding; Stemline Therapeutics: Research Funding. Jain:Incyte: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ravandi:Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; Amgen: Honoraria; AstraZeneca: Consultancy. DiNardo:medimmune: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; jazz: Honoraria; daiichi sankyo: Honoraria; celgene: Consultancy, Honoraria; agios: Consultancy, Honoraria; abbvie: Consultancy, Honoraria; syros: Honoraria. Takahashi:Symbio Pharmaceuticals: Consultancy. Borthakur:Xbiotech USA: Research Funding; Eisai: Research Funding; AstraZeneca: Research Funding; Cantargia AB: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Eli Lilly and Co.: Research Funding; Oncoceutics, Inc.: Research Funding; PTC Therapeutics: Consultancy; NKarta: Consultancy; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cyclacel: Research Funding; GSK: Research Funding; Janssen: Research Funding; Incyte: Research Funding; AbbVie: Research Funding; Merck: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Strategia Therapeutics: Research Funding; Polaris: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; Arvinas: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare AG: Research Funding; Agensys: Research Funding; Oncoceutics: Research Funding; Novartis: Research Funding. Konopleva:Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Agios: Research Funding; Astra Zeneca: Research Funding; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding; Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Kadia:Takeda: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:H3 Biomedicine: Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Merck: Research Funding. Verstovsek:Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Genetech: Research Funding; CTI BioPharma Corp: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Pragmatist: Consultancy; Constellation: Consultancy; Protaganist Therapeutics: Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding. Cortes:Bristol-Myers Squibb: Consultancy, Research Funding; Sun Pharma: Research Funding; Biopath Holdings: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Takeda: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding. Jabbour:AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal