Introduction: The addition of tyrosine kinase inhibitors to hyperfractionated cyclophosphamide, dexamethasone, vincristine, and doxorubicin alternating with high-dose methotrexate and cytarabine (HCVAD) for the treatment of Ph-positive ALL has significantly improved outcomes. However, with the increased median survival, an increased incidence of CNS relapses were documented over time, thus suggesting an increased risk among pts with Ph-positive disease (Ravandi et al, Cancer 2015).In order to reduce this incidence, treatment protocols for Ph-positive ALL were amended in 2012 to increase prophylactic IT chemotherapy from 8 to 12 at our institution. The aim of this study is to compare the incidence of CNS relapses in pts with Ph-positive ALL treated with 8 versus 12 ITs.
Methods: We conducted a retrospective chart review of 156 pts with newly diagnosed Ph-positive ALL treated with Rituximab (R)± HCVAD plus imatinib (n=35), dasatinib (n=68), or ponatinib (n=53) between July 2001 and January 2019. Pts with CNS disease at initial diagnosis were excluded. Complete molecular response (CMR) at 3 months was defined as absence of a quantifiable BCR-ABL1 transcript. CNS relapse was identified by detection of blasts or rare atypical cells in the cerebrospinal fluid (CSF) in at least 2 successive evaluations and/or findings of leptomeningeal disease on imaging. Landmark analysis was performed at 6 months at the approximate time of completion of both systemic and IT therapy. Poor risk cytogenetic abnormality was defined as the presence of +der(22)t(9;22) and/or −9/9p in the absence of high hyperdiploidy (51‐65 chromosomes). CNS relapse-free survival (RFS) was defined from the start of therapy to the time of CNS relapse. Patients who died or relapsed in bone marrow were censored at the time of death and systemic relapse, respectively. Survival was assessed with and without the censoring of allogeneic stem cell transplantation (ASCT).
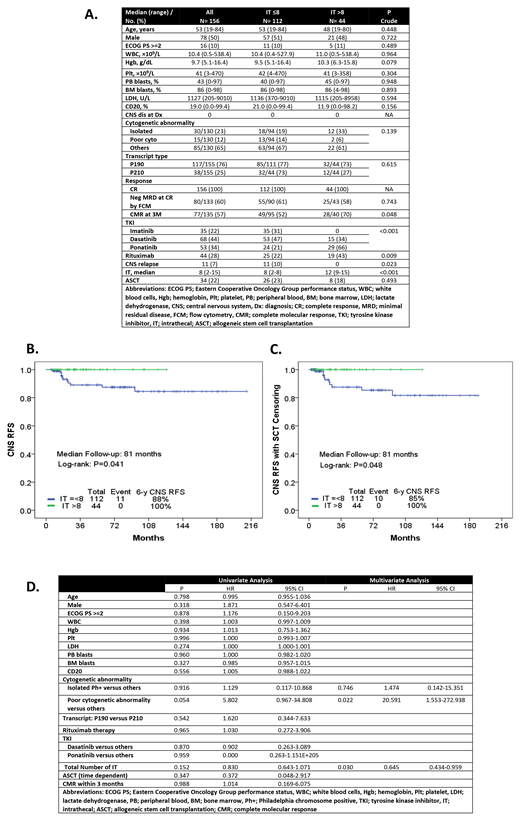
Results: Pt characteristics are summarized in Figure A. One hundred and twelve pts (72%) received a median of 8 ITs (range, 2-8) and 44 pts (28%) received a median of 12 (range, 9-15). There were no statistically significant differences between groups in regards to baseline characteristics with the exception that more patients in the > 8 ITs cohort received ponatinib (66% vs 21%) and thus achieved a higher rate of 3-month CMR (70% vs 52%; p=0.04). CNS relapses were identified in 11 pts overall (7%, 4 treated with imatinib and 7 with dasatinib) and all of them received 8 or less prophylactic ITs (IT ≤8, 10% vs IT >8, 0%; p=0.023).
The median follow-up of the entire population was 81 months, and 97 and 43 months for pts who received ≤8 and >8 ITs, respectively. The 3 and 6-year CNS RFS was 89% and 88% in pts with ≤8 ITs and 100% in pts with >8 Its, respectively (overall P=0.041; 3-yr CNS RFS P=0.049; 6-yr CNS RFS P=0.045) (Figure B). The outcomes remained statistically significant even after censoring for ASCT (P=0.048) (Figure C). In a multivariate analysis and after adjusting for the follow-up time, a median of 12 prophylactic IT chemotherapies was a prognostic factor significantly associated with a decrease rate of CNS relapses (P=0.03; HR=0.64 95%, CI: 0.43-0.96) (Figure D).
Conclusion: In pts with newly diagnosed Ph-positive ALL, incorporation of 12 prophylactic IT chemotherapy in addition to systemic therapy is a very effective strategy to reduce the long-term incidence of CNS relapses.
Paul:Pfizer: Consultancy; Agios: Consultancy. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Kadia:BMS: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioline RX: Research Funding; Celgene: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Garcia-Manero:Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Amphivena: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Ravandi:Macrogenix: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding. Kantarjian:BMS: Research Funding; Novartis: Research Funding; AbbVie: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ariad: Research Funding; Daiichi-Sankyo: Research Funding; Takeda: Honoraria; Pfizer: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Astex: Research Funding; Jazz Pharma: Research Funding; Cyclacel: Research Funding; Immunogen: Research Funding; Amgen: Honoraria, Research Funding. Jabbour:AbbVie: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal