Background: Anemia and thrombocytopenia are common in myelofibrosis (MF), and portend adverse outcomes. Few effective modalities to address these cytopenias exist, particularly thrombocytopenia. Further, although the FDA-approved JAK1/2 inhibitor Ruxolitinib (RUX) has demonstrated significant clinical efficacy in MF patients, RUX frequently causes anemia and thrombocytopenia. Thrombocytopenia, in particular, often results in dose attenuation of RUX. Thalidomide (THAL) is a first-in-class immunomodulatory agent. Studies of THAL in MF patients, both alone and with prednisone, have demonstrated improvements in anemia and thrombocytopenia. We therefore sought to examine whether combination of RUX and THAL could improve both disease-related and therapy-related cytopenias, as well as improve overall disease response in patients with MF. Here we report updated analysis of this study (NCT03069326).
Methods: We conducted a multicenter, two-stage, phase II trial designed to assess the effect of combined RUX and THAL in subjects with primary, post-polycythemia vera, or post-essential thrombocythemia myelofibrosis. Patients taking RUX at the time of enrollment must have had a suboptimal response (less than PR per IWG-MRT/ELN 2013 criteria) or be refractory to RUX single-agent therapy. Patients must have been taking RUX for a minimum of 3 months, and on a stable dose of RUX for a minimum of 4 weeks prior to enrollment. Treatment-naïve patients received single-agent RUX for 3 months (run-in phase) per label, and went on to combination therapy if they achieved less then a PR per IWG-MRT/ELN criteria. Each cycle of therapy was 28 days. Response assessment was evaluated according to the IWG-MRT/ELN 2013 criteria. Platelet response criteria (in patients with baseline thrombocytopenia) included: Major response (≥75% increase in platelet count), Intermediate Response (50-74% increase) and Minor Response (25-49% increase). Adverse events were assessed using the NCI CTCAE v. 4.0. The primary endpoint was the proportion of treated subjects that achieved a response by IWG-MRT criteria and by platelet response criteria.
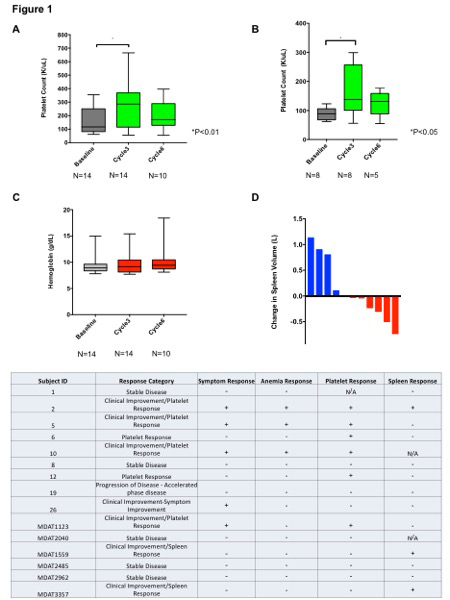
Results: 23 patients have been accrued (planned total accrual 25), 15 of whom are evaluable for response at the time of this writing. The median age was 70 (43-77). 8 patients had received prior therapies other than RUX, including momelotinib, danazol, pomalidomide, darbepoetin alpha and sotatercept. 10 patients enrolled to the run-in phase. 12 patients had received RBC transfusions prior to study enrollment. A significant increase in platelet count after cycle 3 of combination therapy in evaluable patients, compared to baseline, was observed (Figure 1A; P<0.01.) Further, patients with baseline thrombocytopenia experienced a significant increase in platelet count after cycle 3 of therapy (Figure 1B; P<0.05). A trend toward increase in Hgb was observed over successive cycles of combination therapy (Figure 1C). Spleen volume reduction was observed in 6/11 (54.5%) of patients with CT/MRI data available, after at least 3 cycles of combination therapy (Figure 1D). The overall response rate in evaluable patients was 9/15 patients (60%). Clinical Improvement (Anemia response, Symptom response, or Spleen response) occurred in 7/15 patients (46.6%; Table1). Platelet response was observed in 6 of 8 (75%) patients with baseline thrombocytopenia. 1 patient progressed to accelerated phase on study. Grade 3/4 non-hematologic adverse events regardless of attribution included; limb edema, diverticulitis, hypertension, syncope. 1 patient each experienced a thromboembolic event and grade 3 neutropenia.
Conclusions: The combination of THAL and RUX continues to demonstrate a promising efficacy signal in this analysis of an ongoing phase II study, and appears to be well tolerated. Reductions in spleen size and symptom burden were observed. Platelet count increases were observed in 75% patients with baseline thrombocytopenia. This result is particularly important given the lack of treatment modalities for thrombocytopenia in this patient population, and the constraints thrombocytopenia places on RUX dosing, optimization of which is important for spleen response and survival. These data indicate a potential role for this regimen in patients with cytopenias and/or persistent disease manifestations while on RUX. Updated data on responses rate in all accrued patients will be presented.
Rampal:Agios, Apexx, Blueprint Medicines, Celgene, Constellation, and Jazz: Consultancy; Constellation, Incyte, and Stemline Therapeutics: Research Funding. Verstovsek:Astrazeneca: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy; Incyte: Research Funding. King:Incyte: Other: Advisory Board; Genentech: Other: Advisory Board ; Astrazeneca: Other: Advisory board. Stein:Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Pemmaraju:Stemline Therapeutics: Consultancy, Honoraria, Research Funding; cellectis: Research Funding; celgene: Consultancy, Honoraria; affymetrix: Research Funding; sagerstrong: Research Funding; Daiichi-Sankyo: Research Funding; plexxikon: Research Funding; novartis: Consultancy, Research Funding; incyte: Consultancy, Research Funding; samus: Research Funding; abbvie: Consultancy, Honoraria, Research Funding; mustangbio: Consultancy, Research Funding. Mauro:Novartis Oncology: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Pfizer: Consultancy; Takeda: Consultancy. Kadia:AbbVie: Consultancy, Research Funding; Celgene: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioline RX: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding. Bose:CTI BioPharma: Research Funding; Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding; Blueprint Medicine Corporation: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Constellation: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal