Background: Ruxolitinib (rux), a Janus kinase 1/2 inhibitor (JAKi), is the only approved therapy for myelofibrosis (MF), a myeloproliferative neoplasm associated with bone marrow (BM) fibrosis. Rux reduces spleen volume (SVR35 in 30%-40% pts) and constitutional symptoms (≥50% in about 40% pts), two important hallmarks of MF; however, the improvement may be associated with significant cytopenia and rarely with evidence of disease modification. Synergistic therapeutic agents are needed for disease-modifying effects leading to overall improvement of MF, an unmet medical need. BET proteins are transcriptional regulators that control key oncogenic pathways, including NFκB, and TGFβ signaling, important drivers of inflammation and fibrosis, respectively, in MF. In preclinical MF models, the combination of a BETi and rux demonstrated synergistic reduction of splenomegaly, cytokine (Ck) expression, BM fibrosis and the mutant allele burden (Kleppe 2018). CPI-0610 is a selective and potent oral small molecule BETi with effects on megakaryocyte differentiation and Ck production in preclinical studies (unpublished data) and has shown antitumor activity and a wide therapeutic window in a Phase 1 lymphoma study (Blum KA, 2018). Preliminary clinical data from the Phase 2 MANIFEST study in prior JAKi treated MF pts showed that CPI-0610, as monotherapy (Arm 1) or "add on" to rux (Arm 2), was generally well-tolerated, with spleen volume reduction, symptom alleviation, hemoglobin improvement and reduction in transfusion burden as well as suppression in proinflammatory Ck and improvement in BM fibrosis (Kremyanskaya, 2019; Hoffman, 2019). Here, we present preliminary clinical data from Arm 3 in the MANIFEST study: JAKi naïve MF pts treated with CPI-0610 in combination with rux.
Method: MANIFEST is a global, multicenter, open label Phase 2 study of CPI-0610 in combination with rux. Key eligibility criteria of Arm 3 include JAKi naïve MF pts with DIPSS score int-1 or higher, ECOG performance status ≤2, platelet counts ≥100 x 109/L, peripheral blood blast count <10%, anemia (hemoglobin < 10g/dL), ≥5 cm palpable spleen, ≥2 symptoms measurable (score ≥3) or a total symptom score (TSS) of ≥10 using the MFSAF v4.0. Primary endpoint: spleen volume response (SVR); key secondary endpoints: change in TSS, safety and PK; additional endpoints: changes in proinflammatory Ck levels, BM morphology and mutant allele burden.
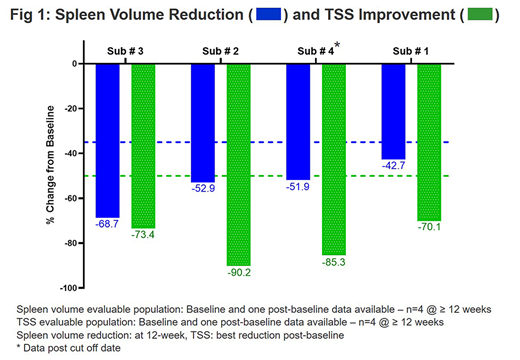
Results: As of 27 June 2019, total 11 pts have been treated, all pts remain on study, 4 pts on treatment for ≥ 4 cycles (≥ 12 weeks). Baseline median age: 71 years (52-76), gender: 8 male (72.7%), ECOG ≤1: 10 (90.9%) pts, primary MF: 8 (72.7%) pts, DIPSS score: int-1/int-2/high: 2/7/2 pts, median platelet: 368 x 109/L (112-951), 9 (81.8%) pts with hemoglobin <10 g/dL, median spleen volume: 1379 cc (580-2807), median TSS: 11.8 (4.1-17), driver mutations: 11 (100%) with ≥1 JAK2/MPL/CALR mutations, HMR (high molecular risk) mutations: 6 (56%) pts, and ≥3 mutations: 4 (36%) pts. All 4 (100%) pts on treatment for ≥ 12 weeks achieved ≥35% spleen volume reduction (median: -52.4%, [range -68.7%, -42.7%]) and all 4 pts (100%) achieved ≥50% improvement in TSS (median best change: -79.35% [range -90.2%, -70.1%]) (Fig. 1). Suppression of proinflammatory Ck, including IL-8, IL-18 and CRP, was also observed. Safety data from the first 6 patients who received treatment for at least 1 cycle were reviewed: no DLTs or grade ≥3 thrombocytopenia was observed. As of 27 June 2019, the most common treatment-emergent adverse events (TEAE) observed in ≥ 2 pts include anemia (1 grade 3), fatigue (all ≤ grade 2), and non-cumulative reversible thrombocytopenia (all ≤ grade 2).
Conclusions: The combination of BETi CPI-0610 and JAKi rux was generally well-tolerated demonstrating that the safety of this combination is acceptable in JAKi naïve MF pts with anemia. Early clinical activity was observed with the combination: all 4 evaluable pts achieved both ≥35% SVR and ≥50% improvement in TSS as early as 3 months after treatment. Available data in JAKi naïve anemic MF pts, a population with poor prognosis, along-with additional information on reduction in pro-inflammatory Ck and BM fibrosis improvement in CPI-610 treated pts in rux refractory MF, collectively indicate that addition of CPI-0610 to rux may be synergistic and potentially have disease-modifying effects in JAKi naïve MF pts. Updated data will be presented.
Harrison:Incyte: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Shire: Speakers Bureau; Roche: Honoraria; Sierra Oncology: Honoraria; CTI: Speakers Bureau; Gilead: Speakers Bureau; Janssen: Speakers Bureau; Promedior: Honoraria; Celgene: Honoraria, Speakers Bureau; AOP: Honoraria. Mascarenhas:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Roche: Consultancy, Research Funding; Merck: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Promedior: Research Funding; Merus: Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kremyanskaya:Incyte, Celgene, Constellation, Protagonist.: Research Funding; La Jolla: Consultancy. Hoffman:Merus: Research Funding. Schiller:Agios: Research Funding, Speakers Bureau; Genzyme: Research Funding; Astellas: Research Funding; Biomed Valley Discoveries: Research Funding; Bristol Myer Squibb: Research Funding; Celgene: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding; Amgen: Other, Research Funding. Leber:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kabir:Constellation Pharmaceuticals: Employment. Senderowicz:Constellation Pharmaceuticals: Employment; Puma Biotechnology: Membership on an entity's Board of Directors or advisory committees. Mertz:Constellation Pharmaceuticals: Employment, Equity Ownership. Trojer:Constellation Pharmaceuticals: Employment, Equity Ownership. Shao:Constellation Pharmaceuticals: Employment. Gupta:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Research Funding; Sierra Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal