JNJ-68284528 (JNJ-4528) is a chimeric antigen receptor T cell (CAR-T) therapy containing two BCMA-targeting single-domain antibodies designed to confer avidity. A first-in-human phase 1 study (LEGEND-2) conducted in China of LCAR-B38M, an identical CAR to JNJ-4528, showed high overall response and manageable safety in 74 patients (pts) with R/R MM. Phase 1b results from the ongoing CARTITUDE-1 study conducted in the US with JNJ-4528 are presented here (NCT03548207).
Eligible pts (≥18 years) were diagnosed with MM per International Myeloma Working Group (IMWG) criteria, had measurable disease as assessed by M-protein or serum free light chain levels, received ≥3 prior regimens or were double refractory to a proteasome inhibitor (PI) and immunomodulatory drug (IMiD), and received an anti-CD38 antibody. Bridging therapy was allowed after apheresis. Cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days were used as the conditioning regimen. A single infusion of JNJ-4528 at the targeted 0.75x106 CAR+ cells/kg (target range 0.5-1.0x106) dose was administered 5-7 days after the start of the conditioning regimen. Primary objectives for phase 1b were to characterize the safety of JNJ-4528 and confirm the recommended phase 2 dose (RP2D).
Adverse events (AEs) were graded using CTCAE, v5.0, cytokine release syndrome (CRS) using Lee et al. (Blood 2014;124:188), and neurotoxicity using both CTCAE, v5.0 and the ASTCT grading system (Lee et al.Biol Blood Marrow Transplant 2019 25(4):625). Response was assessed per IMWG criteria, and minimal residual disease (MRD) was assessed by next generation flow cytometry and/or next generation sequencing.
As of 24 Jun 2019, 25 pts had been infused with JNJ-4528 in the phase 1b portion of the study. Median age was 61 years (range 50-75), pts had received a median of 5 (range 3-16) prior lines of treatment, 88% were triple-refractory to a PI, IMiD, and anti-CD38 antibody, 72% were penta-exposed, and 36% were penta-refractory. The median administered dose was 0.73x106 CAR+ cells/kg (range 0.5-0.9x106).
Most frequently reported AEs were CRS (88%), neutropenia (80%), anemia (76%), and thrombocytopenia (72%). Hematologic AEs of grade ≥3 included neutropenia (76%), thrombocytopenia (60%), and anemia (48%). The majority of pts (80%) had grade 1-2 CRS, with 1 grade 3 event and 1 grade 5 event at day 99 from sequalae of grade 4 CRS (dose-limiting toxicity).
CRS events had a generally predictable time to onset, occurring at a median of 7 days (range 2-12) post-infusion with a median duration of 4 days (range 1-60). Tocilizumab and corticosteroids were administered in 91% and 27% of pts with CRS (n=22), respectively. Three pts had CAR-T-related neurotoxicity of grade 1 (n=2) and grade 3 (n=1); all events occurred in the context of CRS and resolved within 1-2 days.
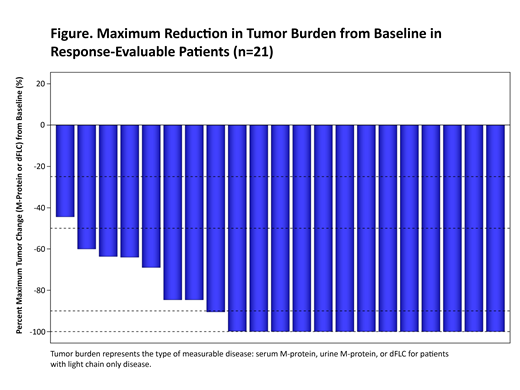
At data cut-off, 21 pts were evaluable for response (postbaseline evaluation at day 28) with a median follow-up of 3 months (range 1-10). Reduction in tumor burden was observed for all pts (Figure). An overall response rate of 91% was observed, with 4 stringent complete responses (sCRs), 2 CRs, 7 very good partial responses, and 6 partial responses. Of the 15 pts with post-infusion day 28 evaluable bone marrow (BM) samples, 10 were MRD-negative at the 10-5 sensitivity level, 2 at the 10-4 sensitivity level, and 3 had unidentified clones. No pts had progressed at the time of data cutoff. Responses were independent of baseline BCMA expression.
JNJ-4528 CAR+ cellular and transgene levels showed expansion and persistence in both blood and BM, with peak expansion 9-14 days after dosing in a majority of pts. All pts showed similar kinetics of decline in soluble BCMA (sBCMA) levels, and continued depletion in sBCMA suggests CAR-T-mediated pharmacodynamic activity. Serum cytokine levels (i.e., IL-6, IFNγ, IL-10) increased post-infusion and peaked around day 10, coinciding with peak expansion of CAR+ T cells. Increases in some proinflammatory cytokines (i.e., IL-6) correlated with onset of CRS symptoms.
Collectively these results demonstrate that JNJ-4528 at a target dose of 0.75x106 CAR+ cells/kg delivers early and deep responses, including MRD negativity in all evaluable pts tested, with a manageable safety profile in pts with refractory MM. The safety and efficacy results from the ongoing CARTITUDE-1 study are consistent with the LEGEND-2 study and confirm the 0.75x106 CAR+ cells/kg dose as the RP2D for further clinical development.
Madduri:AbbVie: Consultancy; Foundation Medicine: Consultancy; Celgene: Consultancy; Takeda: Consultancy. Usmani:Amgen Array Biopharma, Bristol-Myers Squibb, Celgene, Janssen, Merck, Pharmacyclics, Sanofi, Takeda: Other: Research Grant; Amgen, Celgene, Janssen, Sanofi, Takeda: Speakers Bureau; Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, SkylineDX, Takeda: Other: Consultant/Advisor. Jagannath:AbbVie: Consultancy; Karyopharm Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene Corporation: Consultancy; Janssen Pharmaceuticals: Consultancy; Merck & Co.: Consultancy. Singh:Janssen R&D: Employment, Equity Ownership. Zudaire:Janssen R&D: Employment, Equity Ownership. Yeh:Janssen R&D: Employment, Equity Ownership. Allred:Janssen R&D: Employment, Equity Ownership. Banerjee:Janssen R&D: Employment, Equity Ownership. Goldberg:Janssen R&D: Employment, Equity Ownership. Schecter:Janssen R&D, LLC: Employment, Equity Ownership. Zhuang:Janssen R&D: Employment, Equity Ownership. Infante:Janssen R&D: Employment, Equity Ownership. Rizvi:Legend Biotech: Employment, Equity Ownership. Fan:Legend Biotech: Employment, Equity Ownership. Jakubowiak:Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; SkyLineDx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria; KaryoPharm Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Berdeja:Amgen Inc, BioClinica, Celgene Corporation, CRISPR Therapeutics, Bristol-Myers Squibb Company, Janssen Biotech Inc, Karyopharm Therapeutics, Kite Pharma Inc, Prothena, Servier, Takeda Oncology: Consultancy; AbbVie Inc, Amgen Inc, Acetylon Pharmaceuticals Inc, Bluebird Bio, Bristol-Myers Squibb Company, Celgene Corporation, Constellation Pharma, Curis Inc, Genentech, Glenmark Pharmaceuticals, Janssen Biotech Inc, Kesios Therapeutics, Lilly, Novartis, Poseida: Research Funding; Poseida: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal