INTRODUCTION: Overexpression of CD123, the alpha subunit of the IL-3 receptor, is seen in multiple hematological malignancies, including AML, BPDCN, and ALL. IMGN632 is a CD123-targeting ADC, comprising a high affinity anti-CD123 antibody coupled to a DNA-alkylating payload of the novel IGN (indolinobenzodiazepine pseudodimer) class.
METHODS: Adult patients with CD123-positive R/R AML or R/R BPDCN with no more than three prior lines of therapy, were eligible. IMGN632 was given in two schedules: A) dosing day 1 and B) fractionated dosing on days 1, 4, and 8, both on a 21-day cycle.
RESULTS: 74 patients (67 AML, 7 BPDCN) have received IMGN632 across nine dose-escalation cohorts on two schedules, with dosing escalated from 0.015-0.45 mg/kg on schedule A (n=61) and 0.015-0.06 mg/kg on days 1, 4, and 8 on schedule B (n=13). The median age of patients was 69 years (range 33-83). Forty-four percent had secondary AML and 70% of classifiable AML patients were ELN adverse risk (32/46). Twenty-six percent were primary refractory to frontline therapy, 32% were enrolled in first relapse, and 41% had other relapsed-refractory disease. Sixty-eight percent of patients had received prior intense therapy, including stem cell transplant in 19%.
The most common treatment-emergent adverse events (AEs) were diarrhea (30%; all ≤ grade 2), febrile neutropenia (27%; all grade 3), nausea (26%; one grade 3), chills (23%; all ≤ grade 2), and lung infection (22%; ≥ grade 2). The principal treatment-related AEs were infusion-related reactions (16%; four grade 3), which included chills, nausea, diarrhea and tachycardia. None requiring treatment discontinuation. The majority of patients received premedication with dexamethasone. Three dose limiting toxicities occurred at dose levels ≥ 0.18 mg/kg (all in schedule A), including one prolonged neutropenia and two reversible veno-occlusive disease cases. Importantly, no patterns of hepatotoxicity or cytopenias occurred with doses below 0.18 mg/kg, which are doses being evaluated for combinations. The 30-day mortality from last dose was 8%.
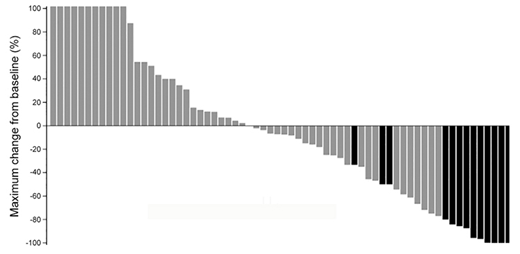
In the assessable AML population (n=66), 37 (55%) had a reduction in bone marrow blasts, and 13 (20%) achieved an objective response (3 CR, 8 CRi, 2 MLFS; Figure 1) across a wide range of doses (0.045 to 0.3 mg/kg). Of note, the majority of responders (77%) had failed prior intensive therapies (including three with prior transplant), 62% had adverse ELN risk classification (including complex karyotype, ASXL1, RUNX1, and FLT3-ITD mutations), and 23% were primary refractory.
Although no MTD was determined on either schedule, based on the efficacy, safety, and PK data, the recommended phase 2 dose (RP2D) and schedule was 0.045 mg/kg given on day 1 every 21 days. In addition to a lack of significant toxicities seen with repeated dosing, and consistency of post-infusion exposures and CD123 saturation, encouraging single agent activity was seen at this level in non-secondary AML patients with a response rate of 32% (6/19; 2 CR, 3 CRi, 1 MLFS).
Of seven R/R BPDCN patients, three (43%) achieved an objective responses (CR, CRi, PR), two others had stable disease and two had clinical progression. The patient with CR had previously had a partial response to SL-401, responded to CHOP, received a transplant and was refractory to decitabine with venetoclax: on IMGN632 this patient cleared bone marrow (28% to 0%) with one dose, and cleared skin (biopsy negative) and CT lesions with 2 doses. The patient with a CRi was refractory to SL-401, CLAG-M, and CLAG, and cleared bone marrow (37% to 0%), skin and CT lesions after one 0.045 mg/kg dose of IMGN632. The patient with a PR had previously had a partial response to SL-401: on IMGN632 this patient had complete clearance of bone marrow blasts (87% to 0%) and significant improvement in skin and CT lesions with one dose of IMGN632.
CONCLUSIONS: In this phase 1 trial, the novel CD123-targeting ADC IMGN632 demonstrated a manageable safety profile and broad therapeutic window in high risk R/R AML and BPDCN patients. The encouraging single agent activity and safety profile, along with supporting preclinical efficacy studies, have led to the subsequent development of IMGN632 as monotherapy in patients with R/R BPDCN and MRD+ AML, and in combination with azacitidine and/or venetoclax in patients with R/R and untreated AML.
Figure 1. Maximum % decrease in bone marrow blasts from baseline. Objective responses (CR, CRi, or MLFS) are shown in black.
Daver:Glycomimetics: Research Funding; Otsuka: Consultancy; Pfizer: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Celgene: Consultancy; Hanmi Pharm Co., Ltd.: Research Funding; Karyopharm: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Servier: Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding; Jazz: Consultancy; Karyopharm: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Astellas: Consultancy; Astellas: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Forty-Seven: Consultancy; Forty-Seven: Consultancy; Jazz: Consultancy; Glycomimetics: Research Funding; Incyte: Consultancy, Research Funding; Otsuka: Consultancy; Sunesis: Consultancy, Research Funding; NOHLA: Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Agios: Consultancy; Servier: Research Funding; NOHLA: Research Funding; Celgene: Consultancy. Montesinos:Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research support, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research support, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Teva: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: Research support; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees. DeAngelo:Glycomimetics: Research Funding; Blueprint: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Abbvie: Research Funding; Amgen, Autolus, Celgene, Forty-seven, Incyte, Jazzs, Pfizer, Shire, Takeda: Consultancy. Wang:Abbvie: Other: Advisory role; Kite: Other: Advisory role; Jazz: Other: Advisory role; Astellas: Other: Advisory role, Speakers Bureau; celyad: Other: Advisory role; Pfizer: Other: Advisory role, Speakers Bureau; Stemline: Other: Advisory role, Speakers Bureau; Daiichi: Other: Advisory role; Amgen: Other: Advisory role; Agios: Other: Advisory role. Papadantonakis:Agios: Consultancy, Honoraria. Erba:Incyte: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy; Pfizer: Consultancy; Seattle Genetics: Consultancy; Celgene: Consultancy, Other: chair, AML Registry Scientific Steering Committee, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Covance: Other: Fees for serving as chair on an independent review board for AbbVie Phase III studies; GlycoMimetics: Consultancy, Other: Chair, data and safety monitoring board, Research Funding; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; MacroGenics: Consultancy, Other: Lecture fees, Research Funding; Pfizer: Consultancy; Celgene: Consultancy, Other: chair, AML Registry Scientific Steering Committee, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; Agios: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; GlycoMimetics: Consultancy, Other: Chair, data and safety monitoring board, Research Funding; Agios: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy; Novartis: Consultancy, Research Funding, Speakers Bureau; MacroGenics: Consultancy, Other: Lecture fees, Research Funding; Amgen: Consultancy; Astellas Pharma: Consultancy; Astellas Pharma: Consultancy; ImmunoGen: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Covance: Other: Fees for serving as chair on an independent review board for AbbVie Phase III studies; AbbVie: Consultancy, Other: Chair, IRC for phase III studies, Research Funding; AbbVie: Consultancy, Other: Chair, IRC for phase III studies, Research Funding. Pemmaraju:cellectis: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; novartis: Consultancy, Research Funding; incyte: Consultancy, Research Funding; celgene: Consultancy, Honoraria; samus: Research Funding; abbvie: Consultancy, Honoraria, Research Funding; mustangbio: Consultancy, Research Funding; affymetrix: Research Funding; sagerstrong: Research Funding; Daiichi-Sankyo: Research Funding; plexxikon: Research Funding. Lane:AbbVie: Research Funding; Stemline Therapeutics: Research Funding; N-of-One: Consultancy. Rizzieri:Celgene, Gilead, Seattle Genetics, Stemline: Other: Speaker; AbbVie, Agios, AROG, Bayer, Celgene, Gilead, Jazz, Novartis, Pfizer, Sanofi, Seattle Genetics, Stemline, Teva: Other: Advisory Board; AROG, Bayer, Celgene, Celltron, Mustang, Pfizer, Seattle Genetics, Stemline: Consultancy; Stemline: Research Funding. Sweet:Agios: Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy; Celgene: Speakers Bureau; Jazz: Speakers Bureau; Incyte: Research Funding; Pfizer: Consultancy; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees. Konopleva:Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; Ascentage: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; Ablynx: Research Funding; Astra Zeneca: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Kisoji: Consultancy, Honoraria; Agios: Research Funding; Calithera: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Sloss:ImmunoGen: Employment. Culm-Merdek:ImmunoGen Inc: Employment. Zweidler-McKay:ImmunoGen: Employment. Kantarjian:AbbVie: Honoraria, Research Funding; Jazz Pharma: Research Funding; Agios: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; BMS: Research Funding; Cyclacel: Research Funding; Daiichi-Sankyo: Research Funding; Ariad: Research Funding; Amgen: Honoraria, Research Funding; Novartis: Research Funding; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; Astex: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal