Key Points
Both watch-and-wait and treated CLL patients have high mortality rates when admitted for COVID-19.
Receiving a BTKi for CLL at COVID-19 diagnosis severe enough to require hospitalization did not influence case fatality rate in this study.
Abstract
Given advanced age, comorbidities, and immune dysfunction, chronic lymphocytic leukemia (CLL) patients may be at particularly high risk of infection and poor outcomes related to coronavirus disease 2019 (COVID-19). Robust analysis of outcomes for CLL patients, particularly examining effects of baseline characteristics and CLL-directed therapy, is critical to optimally manage CLL patients through this evolving pandemic. CLL patients diagnosed with symptomatic COVID-19 across 43 international centers (n = 198) were included. Hospital admission occurred in 90%. Median age at COVID-19 diagnosis was 70.5 years. Median Cumulative Illness Rating Scale score was 8 (range, 4-32). Thirty-nine percent were treatment naive (“watch and wait”), while 61% had received ≥1 CLL-directed therapy (median, 2; range, 1-8). Ninety patients (45%) were receiving active CLL therapy at COVID-19 diagnosis, most commonly Bruton tyrosine kinase inhibitors (BTKi’s; n = 68/90 [76%]). At a median follow-up of 16 days, the overall case fatality rate was 33%, though 25% remain admitted. Watch-and-wait and treated cohorts had similar rates of admission (89% vs 90%), intensive care unit admission (35% vs 36%), intubation (33% vs 25%), and mortality (37% vs 32%). CLL-directed treatment with BTKi’s at COVID-19 diagnosis did not impact survival (case fatality rate, 34% vs 35%), though the BTKi was held during the COVID-19 course for most patients. These data suggest that the subgroup of CLL patients admitted with COVID-19, regardless of disease phase or treatment status, are at high risk of death. Future epidemiologic studies are needed to assess severe acute respiratory syndrome coronavirus 2 infection risk, these data should be validated independently, and randomized studies of BTKi’s in COVID-19 are needed to provide definitive evidence of benefit.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has challenged our health care systems and threatened individuals across the globe, particularly the most vulnerable, including the elderly and those with medical comorbidities. Understanding how severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), the virus responsible for COVID-19, impacts those with preexisting conditions remains critically important as we manage the current outbreak, prepare for potential future waves of COVID-19, and consider the dynamics of postinfection immunity and response to immunization.
Many patients with cancer carry an excess risk of infection from both underlying malignancy and cancer-directed therapy. Further study of COVID-19 in this complex population is underway. An early epidemiologic study of 1099 patients diagnosed with COVID-19 in the first 2 months of the outbreak in Wuhan, China suggested that intensive care unit (ICU) admission was required in 5.0%, use of mechanical ventilation was required in 2.3%, and death occurred in 1.4%. In this study, only 10 patients (0.9%) had a concomitant cancer diagnosis.1 In a subsequent series of 2,007 patients diagnosed with and admitted to a hospital for COVID-19, 18 (1%) had a history of cancer.2 This report suggested that cancer patients had more severe disease with higher rates of ICU admission, requirement for mechanical ventilation, and death.2 In a large report of 72 314 cases in China, the case fatality rate among the subgroup with cancer was 5.6% as compared with 2.3% in the entire population, suggesting that cancer patients may suffer a more aggressive clinical course.3
As the pandemic has spread, international reports have confirmed the gravity of this illness. Of 5700 patients hospitalized with COVID-19 in New York City, 14.2% required ICU admission, 3.2% required intubation, and 21% died. In this series, 6% carried a cancer diagnosis, though mortality of this subgroup was not reported.4 Reports from other medical centers in New York City have reported case fatality rates ranging from 10.2% to 22% in admitted patient populations.5-7 A UK analysis of 16 749 hospitalized patients showed an overall mortality rate for all inpatients of 33%, and those with a history of cancer had an increased risk of death due to COVID-19 compared with those without (odds ratio, 1.19; 95% confidence interval [CI], 1.03-1.38; P = .019).8 The recently published UK Coronavirus Cancer Monitoring Project described outcomes of 800 cancer patients, including a case fatality rate of 28% in a predominantly inpatient cohort (88%) and no significant effect of cancer treatment within 4 weeks on case fatality rate.9
Even among patients with cancer, the overall prognosis, degree of immunodeficiency, and effect of therapy on immunocompetence varies widely, likely leading to very different outcomes for patients diagnosed with COVID-19 across malignancies. These factors make the application of the existing COVID-19 studies challenging and lead to a clear need for robust disease-specific COVID-19 analyses.
Chronic lymphocytic leukemia (CLL) is the most commonly diagnosed leukemia in the Western world.10 Patients with CLL may be at particular risk for COVID-19 and its complications, as CLL is a disease of older people (median age at diagnosis, 70 years)11 and is associated with profound immune dysregulation. The underlying disease process in CLL impacts both humoral and cellular immune function, and several classes of CLL-directed therapy modulate immune response.12 CLL patients are known to carry an excess risk of infection and death due to infection due to both the underlying disease process and CLL-directed therapy.13 In the early stages of this pandemic, CLL-directed care has been profoundly impacted, with professional societies advocating for limited physical exposure to the health care system, delays in therapy, or modification in choice of therapy.14-16 These guidelines, based on expert consensus, were issued out of caution given concern that patients with CLL might have increased risk of SARS-CoV-2 infection and subsequent COVID-19 morbidity and mortality.
Small, heterogeneous case series of patients with hematologic malignancies and SARS-CoV-2 infection have been reported, with minimal information regarding disease status, prior or existing treatment, or histological classification.17 Large, high-quality series of patients with hematological malignancies have been lacking, and there has been limited information on patients with CLL and COVID-19.18-20 A small UK case series has recently reported adverse outcomes in treatment-naive CLL patients, raising the possibly that the “watch and wait” population may be at considerable risk.21 It is unclear to date whether subgroups of CLL patients are at particular risk and whether therapy such as Bruton tyrosine kinase (BTK) inhibition modulates or enhances risk.22-24
Although these recent larger pan-histology cancer series have started to provide some detailed analysis of factors associated with adverse outcome among cancer patients,9,25 it remains unclear whether previously described risk factors (eg, diabetes, chronic renal disease, and age) associated with adverse COVID-19 outcomes8 remain important within the CLL patient population. As uncertainty remains around transmission dynamics and impact on the CLL patient population, data on outcomes for CLL patients who have developed symptomatic COVID-19, particularly the effect of CLL-directed therapy on outcomes, are likely to fundamentally shape how we manage CLL patients as the pandemic continues and evolves.
Methods
In this multicenter, international cohort study, we collected data on all patients with a history of CLL diagnosed with symptomatic COVID-19 (based on presence of SARS-CoV-2 RNA confirmed by reverse transcriptase quantitative polymerase chain reaction) between 17 February 2020 and 30 April 2020 across 43 centers (20 US centers and 23 international centers in the European Union/United Kingdom and South America). This study was institutional review board approved and conducted in accordance with the Declaration of Helsinki.
Investigators at each center collected data using a standardized case report form. Data collected included demographics, baseline characteristics, preexisting comorbidities (including Cumulative Illness Rating Scale [CIRS] score26 and presence or absence of predefined comorbidities), CLL treatment history, details regarding COVID-19 clinical signs and symptoms, COVID-19 management strategies, and clinically relevant outcomes (hospital admission, ICU admission, discharge, and vital status). Data on COVID-19 management strategies collected included use of supplemental oxygen, mechanical ventilation, hemodialysis, antiviral therapies, lopinavir/ritonavir, hydroxychloroquine, remdesivir, corticosteroid, tocilizumab, convalescent plasma, and/or other agents on clinical trials.
The primary study end point was to estimate overall survival (OS) for patients diagnosed with symptomatic COVID-19 during this time period, defined as the time from COVID-19 diagnosis to death. Patients alive at the time of analysis were censored at last follow-up. OS was estimated using the Kaplan-Meier method. Secondary end points were a description of clinical features including baseline characteristics, COVID-19–related symptoms at presentation, CLL treatment history, description of current practices regarding management of COVID-19, and examination of OS stratified by CLL treatment history.
Utilizing Cox regression, univariable analyses were performed to evaluate relationship between baseline characteristics and OS. Significant predictors from univariable analyses (P < .05) were included in a multivariable Cox regression to test for predictors of mortality. Other comorbidities were included in multivariable Cox regression to control for potential confounders. All other comparisons were descriptive. Statistical analyses were performed using Stata 10.1 (Stata Statistical Software, release 10, 2007; StataCorp, College Station, TX). The database was locked on 30 April 2020 for analysis.
Results
Patient characteristics
We identified 198 patients with CLL who were diagnosed with symptomatic COVID-19 between 17 February 2020 and 30 April 2020. The median age at initial diagnosis of CLL was 63 years (range, 35-92 years), and the median age at COVID-19 diagnosis was 70.5 years (range, 38-98 years) (Table 1). The population had a male predominance (63%) and was mostly white (88%). Most cases were diagnosed in the United States or Europe (98%; 50% United States, 29% Spain, 15% United Kingdom, and 4% other European countries). Many patients had a significant burden of comorbidities, with a median CIRS score of 8 (range, 4-32). Hypertension (51%), hypogammaglobulinemia (44%), arrhythmia history (20%), diabetes (20%), COPD/asthma (17%), and chronic renal disease (17%) were common, notable comorbidities. Only 7% of patients were active tobacco smokers, while 66% had never smoked and 27% were former smokers.
Baseline characteristics
| Characteristic . | Entire cohort (n = 198) . | Patients requiring admission (n = 178) . | Patients who died (n = 66) . | |||
|---|---|---|---|---|---|---|
| Proportion (unless otherwise specified) . | Number with available data . | Proportion (unless otherwise specified) . | Number with available data . | Proportion (unless otherwise specified) . | Number with available data . | |
| Age (y) at CLL diagnosis, median (range) | 63 (35-92) | 195 | 63 (35-92) | 175 | 65 (40-92) | 65 |
| Age (y) at COVID-19 diagnosis | 198 | 178 | 66 | |||
| Median (range) | 70.5 (38-98) | 71 (41-98) | 73 (43-98) | |||
| ≥65 y | 67% | 68% | 71% | |||
| ≥75 y | 36% | 37% | 47% | |||
| Male | 63% | 198 | 62% | 178 | 59% | 66 |
| White | 88% | 196 | 90% | 177 | 92% | 65 |
| CIRS | 171 | 156 | 58 | |||
| Median (range) | 8 (4-32) | 8 (4-32) | 9 (4-32) | |||
| >6 | 67% | 67% | 81% | |||
| Comorbidities | ||||||
| Hypertension | 51% | 198 | 51% | 178 | 56% | 66 |
| Coronary artery disease | 13% | 192 | 13% | 172 | 14% | 63 |
| Arrhythmia | 20% | 197 | 23% | 177 | 25% | 65 |
| Diabetes | 20% | 198 | 20% | 178 | 32% | 66 |
| COPD | 11% | 198 | 11% | 178 | 14% | 66 |
| Asthma | 6% | 197 | 7% | 177 | 11% | 65 |
| Chronic renal disease | 17% | 198 | 18% | 178 | 24% | 66 |
| Autoimmune disease | 10% | 198 | 10% | 178 | 9% | 66 |
| Hypogammaglobulinemia | 44% | 177 | 45% | 157 | 36% | 58 |
| Smoking history | 196 | 176 | 66 | |||
| Never smoker | 66% | 65% | 65% | |||
| Former smoker | 27% | 28% | 23% | |||
| Current smoker | 7% | 7% | 12% | |||
| Labs at COVID-19 diagnosis | ||||||
| Absolute neutrophil count (thousand cells/μL), median (range) | 4.6 (0.0-33.5) | 184 | 4.7 (0.3-33.5) | 169 | 4.8 (0.4-25.9) | 61 |
| Absolute lymphocyte count (thousand cells/μL), median (range) | 7.8 (0.0-579) | 185 | 7.8 (0.0-579) | 170 | 11.6 (0.2-253) | 62 |
| CLL treatment history | 195 | 175 | 68 | |||
| Never treated | 39% | 39% | 42% | 66 | ||
| Prior therapy | 61% | 61% | 58% | |||
| Lines of therapy for previously treated patients, median (range) | 2 (1-8) | 119 | 2 (1-8) | 107 | 2 (1-8) | 38 |
| Prior fludarabine or bendamustine | 28% | 183 | 28% | 164 | 26% | 61 |
| Receiving therapy at time of COVID-19 diagnosis | 45% | 198 | 46% | 178 | 38% | 66 |
| Characteristic . | Entire cohort (n = 198) . | Patients requiring admission (n = 178) . | Patients who died (n = 66) . | |||
|---|---|---|---|---|---|---|
| Proportion (unless otherwise specified) . | Number with available data . | Proportion (unless otherwise specified) . | Number with available data . | Proportion (unless otherwise specified) . | Number with available data . | |
| Age (y) at CLL diagnosis, median (range) | 63 (35-92) | 195 | 63 (35-92) | 175 | 65 (40-92) | 65 |
| Age (y) at COVID-19 diagnosis | 198 | 178 | 66 | |||
| Median (range) | 70.5 (38-98) | 71 (41-98) | 73 (43-98) | |||
| ≥65 y | 67% | 68% | 71% | |||
| ≥75 y | 36% | 37% | 47% | |||
| Male | 63% | 198 | 62% | 178 | 59% | 66 |
| White | 88% | 196 | 90% | 177 | 92% | 65 |
| CIRS | 171 | 156 | 58 | |||
| Median (range) | 8 (4-32) | 8 (4-32) | 9 (4-32) | |||
| >6 | 67% | 67% | 81% | |||
| Comorbidities | ||||||
| Hypertension | 51% | 198 | 51% | 178 | 56% | 66 |
| Coronary artery disease | 13% | 192 | 13% | 172 | 14% | 63 |
| Arrhythmia | 20% | 197 | 23% | 177 | 25% | 65 |
| Diabetes | 20% | 198 | 20% | 178 | 32% | 66 |
| COPD | 11% | 198 | 11% | 178 | 14% | 66 |
| Asthma | 6% | 197 | 7% | 177 | 11% | 65 |
| Chronic renal disease | 17% | 198 | 18% | 178 | 24% | 66 |
| Autoimmune disease | 10% | 198 | 10% | 178 | 9% | 66 |
| Hypogammaglobulinemia | 44% | 177 | 45% | 157 | 36% | 58 |
| Smoking history | 196 | 176 | 66 | |||
| Never smoker | 66% | 65% | 65% | |||
| Former smoker | 27% | 28% | 23% | |||
| Current smoker | 7% | 7% | 12% | |||
| Labs at COVID-19 diagnosis | ||||||
| Absolute neutrophil count (thousand cells/μL), median (range) | 4.6 (0.0-33.5) | 184 | 4.7 (0.3-33.5) | 169 | 4.8 (0.4-25.9) | 61 |
| Absolute lymphocyte count (thousand cells/μL), median (range) | 7.8 (0.0-579) | 185 | 7.8 (0.0-579) | 170 | 11.6 (0.2-253) | 62 |
| CLL treatment history | 195 | 175 | 68 | |||
| Never treated | 39% | 39% | 42% | 66 | ||
| Prior therapy | 61% | 61% | 58% | |||
| Lines of therapy for previously treated patients, median (range) | 2 (1-8) | 119 | 2 (1-8) | 107 | 2 (1-8) | 38 |
| Prior fludarabine or bendamustine | 28% | 183 | 28% | 164 | 26% | 61 |
| Receiving therapy at time of COVID-19 diagnosis | 45% | 198 | 46% | 178 | 38% | 66 |
COPD, chronic obstructive pulmonary disease.
Thirty-nine percent (n = 76/195) of patients had never been treated for their CLL (watch and wait), while 61% (n = 119/207) had received ≥1 CLL-directed therapy (median, 2 prior therapies; range, 1-8). Ninety patients (45%) were receiving active CLL-directed therapy at the time of COVID-19 diagnosis, most on novel agents. BTK inhibitors (BTKi’s), either as monotherapy (n = 54) or in combination with other agents (n = 14), were the most common therapy, followed by the BCL-2 inhibitor venetoclax ± anti-CD20 monoclonal antibodies (mAbs; n = 14) (Table 2). A minority of patients were receiving other therapies, including anti-CD20 mAb monotherapies (n = 2), phosphatidylinositol-3-kinase (PI3K) inhibitors (n = 2), a non-BTKi–based novel agent containing combination therapies (n = 1), chemoimmunotherapy combinations (n = 1), or other regimens (n = 2) at the time of COVID-19 diagnosis.
CLL-directed therapy at time of COVID-19 diagnosis
| Current therapy . | Patients receiving therapy . |
|---|---|
| Total | 90 |
| BTKi | |
| Ibrutinib monotherapy | 43 |
| Acalabrutinib monotherapy | 9 |
| Zanubrutinib monotherapy | 2 |
| Ibrutinib + anti-CD20 mAb | 6 |
| Acalabrutinib + anti-CD20 mAb | 1 |
| Venetoclax | |
| Venetoclax monotherapy | 7 |
| Venetoclax + anti-CD20 mAb | 7 |
| PI3K inhibitor | |
| Idelalisib | 1 |
| Umbralisib | 1 |
| Anti-CD20 mAb | |
| Rituximab | 1 |
| Obinutuzumab | 1 |
| Novel drug combination therapy | |
| BTKi + venetoclax | 2 |
| BTKi + venetoclax + anti-CD20 mAb | 1 |
| BTKi + PI3Ki + anti-CD20 mAb | 3 |
| Venetoclax + PI3Ki + anti-CD20 mAb | 1 |
| BTKi + fludarabine + pembrolizumab | 1 |
| Bendamustine + rituximab | 1 |
| Other | 2 |
| Current therapy . | Patients receiving therapy . |
|---|---|
| Total | 90 |
| BTKi | |
| Ibrutinib monotherapy | 43 |
| Acalabrutinib monotherapy | 9 |
| Zanubrutinib monotherapy | 2 |
| Ibrutinib + anti-CD20 mAb | 6 |
| Acalabrutinib + anti-CD20 mAb | 1 |
| Venetoclax | |
| Venetoclax monotherapy | 7 |
| Venetoclax + anti-CD20 mAb | 7 |
| PI3K inhibitor | |
| Idelalisib | 1 |
| Umbralisib | 1 |
| Anti-CD20 mAb | |
| Rituximab | 1 |
| Obinutuzumab | 1 |
| Novel drug combination therapy | |
| BTKi + venetoclax | 2 |
| BTKi + venetoclax + anti-CD20 mAb | 1 |
| BTKi + PI3Ki + anti-CD20 mAb | 3 |
| Venetoclax + PI3Ki + anti-CD20 mAb | 1 |
| BTKi + fludarabine + pembrolizumab | 1 |
| Bendamustine + rituximab | 1 |
| Other | 2 |
Baseline characteristics for the entire cohort (n = 198), as well as the subsets who required hospitalization (n = 178) and those who died due to COVID-19–related complications (n = 66), are described in Table 1. Of note, the proportion of patients with age ≥75 years (P = .02), CIRS score ≥ 6 (P = .006), diabetes (P = .004), or chronic renal disease (P = .04) and who were active smokers (P = .002) was significantly different between the entire cohort and those who died (Table 1).
COVID-19 symptoms and presentation
Data regarding specific symptoms manifested during COVID-19 are outlined in Table 3. All (100%, n = 198) patients in this series had ≥1 clinical symptom; our study did not aim to capture CLL patients who were asymptomatic SARS-CoV-2 carriers. The 5 most common symptoms included fever (88%), cough (85%), fatigue (72%), dyspnea (74%), and myalgia or arthralgia (36%). Fever, cough, and dyspnea were all present in 59% of patients at COVID-19 diagnosis. Rates of anosmia and dysgeusia were not collected.
COVID-19 signs and symptoms
| Symptom . | Proportion (%) . | Number with available data . |
|---|---|---|
| Fever | 88 | 196 |
| Cough | 85 | 193 |
| Sputum production | 25 | 183 |
| Hemoptysis | 2 | 190 |
| Dyspnea | 74 | 197 |
| Nasal congestion | 18 | 183 |
| Sore throat | 16 | 184 |
| Myalgias/arthralgias | 36 | 176 |
| Headache | 16 | 179 |
| Fatigue | 72 | 192 |
| Chills | 34 | 185 |
| Diarrhea | 29 | 190 |
| Nausea/vomiting | 14 | 192 |
| Evidence of DIC | 16 | 184 |
| Lymphopenia (ALC <1.0 × 109/L) | 25 | 185 |
| Symptom . | Proportion (%) . | Number with available data . |
|---|---|---|
| Fever | 88 | 196 |
| Cough | 85 | 193 |
| Sputum production | 25 | 183 |
| Hemoptysis | 2 | 190 |
| Dyspnea | 74 | 197 |
| Nasal congestion | 18 | 183 |
| Sore throat | 16 | 184 |
| Myalgias/arthralgias | 36 | 176 |
| Headache | 16 | 179 |
| Fatigue | 72 | 192 |
| Chills | 34 | 185 |
| Diarrhea | 29 | 190 |
| Nausea/vomiting | 14 | 192 |
| Evidence of DIC | 16 | 184 |
| Lymphopenia (ALC <1.0 × 109/L) | 25 | 185 |
ALC, absolute lymphocyte count; DIC, disseminated intravascular coagulation.
Table 4 outlines observed COVID-19 management strategies. Chest imaging was performed (computed tomography or X-ray) for 94%, of whom 90% had radiographic evidence of pneumonia. Hospital admission occurred in 90% (n = 178) of patients. Of hospital admitted patients, 92% required supplemental oxygen, 38% received ICU-level care, 27% required IV vasopressor support, and 11% required hemodialysis. When comparing watch-and-wait patients with those who had previously received CLL-directed therapy, similar rates of hospital admission (89% vs 90%), ICU admission (35% vs 36%), and intubation (33% vs 25%) were observed.
COVID-19 management
| . | Entire cohort (n = 198) . | Admitted patients (n = 178) . | ||
|---|---|---|---|---|
| . | Proportion (%) . | Number with available data . | Proportion (%) . | Number with available data . |
| Admitted | 90 | 198 | 100 | 178 |
| ICU admission | 35 | 194 | 38 | 178 |
| Imaging performed | 94 | 195 | 97 | 175 |
| Pneumonia on imaging | 90 | 186 | 96 | 171 |
| Supplemental oxygen | 85 | 194 | 92 | 177 |
| Mechanical ventilation | 28 | 190 | 30 | 174 |
| IV vasopressors | 25 | 189 | 27 | 173 |
| Hemodialysis | 10 | 191 | 11 | 175 |
| Agents used for COVID-19 | ||||
| Hydroxychloroquine | 55 | 195 | ||
| Remdesivir | 7 | 195 | ||
| Lopinavir/ritonavir | 17 | 195 | ||
| Tocilizumab | 22 | 195 | ||
| IVIG | 7 | 196 | ||
| Corticosteroids | 48 | 195 | ||
| Azithromycin | 27 | 198 | ||
| Convalescent plasma | 5 | 198 | ||
| . | Entire cohort (n = 198) . | Admitted patients (n = 178) . | ||
|---|---|---|---|---|
| . | Proportion (%) . | Number with available data . | Proportion (%) . | Number with available data . |
| Admitted | 90 | 198 | 100 | 178 |
| ICU admission | 35 | 194 | 38 | 178 |
| Imaging performed | 94 | 195 | 97 | 175 |
| Pneumonia on imaging | 90 | 186 | 96 | 171 |
| Supplemental oxygen | 85 | 194 | 92 | 177 |
| Mechanical ventilation | 28 | 190 | 30 | 174 |
| IV vasopressors | 25 | 189 | 27 | 173 |
| Hemodialysis | 10 | 191 | 11 | 175 |
| Agents used for COVID-19 | ||||
| Hydroxychloroquine | 55 | 195 | ||
| Remdesivir | 7 | 195 | ||
| Lopinavir/ritonavir | 17 | 195 | ||
| Tocilizumab | 22 | 195 | ||
| IVIG | 7 | 196 | ||
| Corticosteroids | 48 | 195 | ||
| Azithromycin | 27 | 198 | ||
| Convalescent plasma | 5 | 198 | ||
IVIG, IV gammaglobulin.
Survival outcomes
As of 30 April 2020, 66 deaths were observed (33% case fatality rate) for this population identified with symptomatic COVID-19. For those who required hospital admission, the case fatality rate was 37%. The small subset of patients who were not admitted to this hospital were younger and less comorbid (supplemental Table 2, available on the Blood Web site); the case fatality rate in this group was 5% (1/20). At this time, mortality for patients requiring supplemental oxygen, admitted to the ICU, or requiring intubation and mechanical ventilation were 39%, 43%, and 55%, respectively. Case fatality rates are likely to be underestimates, as 49 patients are alive but remain hospitalized at the time of this analysis. Of the 129 patients who were admitted and discharged or died (ie, their COVID-19 disease course has neared or reached completion), the overall case fatality rate was 50% (65/129).
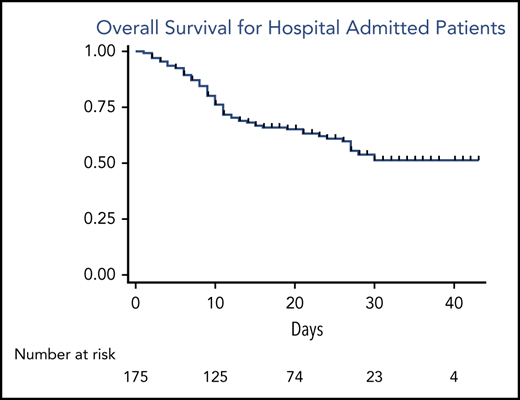
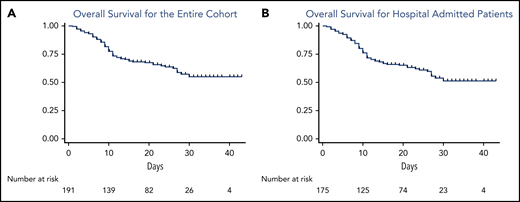
Figure 1 describes OS for the entire study cohort (Figure 1A) and is restricted to patients requiring inpatient admission with symptomatic COVID-19 (Figure 1B). The median follow-up at the time of this analysis was 16 days (range, 1-43 days), representing a total of 3380 days at risk. The 14-day and 28-day OS estimates were 71% and 63%, respectively. Case fatality rates were similar for watch-and-wait patients and patients who had ever received CLL-directed therapy (37% vs 32%). Mortality rate for patients on active CLL-directed therapy at the time of COVID-19 diagnosis was 28%. To assess for potential differences in available health care resources, we assessed case fatality rates for admitted patients by country of diagnosis (United States, 34%; Spain, 42%; and United Kingdom, 33%). In addition, OS was similar when stratified by the number of cases contributed per center (≤5 cases vs >5 cases; supplemental Figure 1).
OS from the time of COVID-19 diagnosis of the entire cohort and admitted patients. (A) OS for the entire cohort; (B) OS for admitted patients.
OS from the time of COVID-19 diagnosis of the entire cohort and admitted patients. (A) OS for the entire cohort; (B) OS for admitted patients.
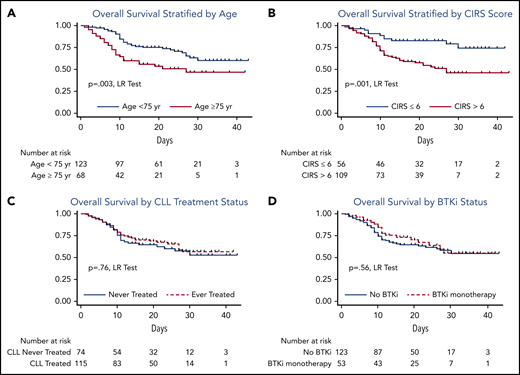
Table 5 describes univariable analysis of baseline characteristics as predictors of OS. CIRS score (>6 vs ≤6), age at COVID-19 diagnosis (≥75 vs <75 years), smoking history (current vs never/former smoker), underlying asthma, diabetes, and chronic renal disease were associated with an increased risk of mortality. Watch-and-wait patients had similar risk of death as patients who had received prior CLL-directed therapy and those who were on active therapy at the time of the event. Even when adjusted for age and CIRS score, having received CLL-directed therapy did not impact OS (hazard ratio [HR], 0.77; 95% CI, 0.47-1.26; P = .30). We did not observe a clear protective or adverse effect of BTKi therapy when compared with patients not receiving BTKi’s, even when adjusting for age, CIRS score, and the number of prior therapies (adjusted HR, 0.92; 95% CI, 0.52-1.59; P = .75). When these analyses were performed to include only the 173 patients requiring admission, survival was similar for those who had been treated vs “watch-and-wait patients (HR, 0.83; 95% CI, 0.51-1.36; P = .47) and those on BTKi’s vs not on BTKi’s at the time of COVID-19 diagnosis (HR, 0.73; 95% CI, 0.43-1.26; P = .27). In multivariable analyses of significant predictors from univariable analyses (excluding smoking status given the small number of active smokers; Table 5), age ≥75 years (HR, 1.8; 95% CI, 1.1-3.0; P = .028), CIRS score >6 (HR, 1.6; 95% CI, 1.0-2.9; P = .043), asthma (HR, 2.5; 95% CI, 1.1-5.8; P = .025), and chronic renal disease (HR, 1.8; 95% CI, 1.0-3.4; P = .025) remained predictors of inferior OS. Figure 2 depicts OS from time of COVID-19 diagnosis stratified by age, CIRS score, treatment history, and use of BTKi’s at time of COVID-19 diagnosis.
Univariable and multivariable analyses of baseline characteristics as predictors of OS
| . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Univariable analyses | |||
| Sex (male vs female) | 0.66 | 0.40-1.1 | .10 |
| Age at COVID-19 diagnosis (≥75 vs <75 y) | 2.0 | 1.2-3.3 | .004 |
| CIRS (>6 vs ≤6) | 2.8 | 1.4-5.4 | .001 |
| Lymphopenia (ALC ≥1.0 × 109/L vs ALC <1.0 × 109/L) | 1.0 | 0.58-1.9 | .88 |
| Comorbidities | |||
| Hypertension | 1.4 | 0.88-2.4 | .137 |
| Diabetes | 2.0 | 1.2-3.3 | .011 |
| Arrhythmia | 1.5 | 0.87-2.7 | .13 |
| Coronary artery disease | 1.3 | 0.64-2.7 | .44 |
| COPD | 1.4 | 0.67-2.7 | .40 |
| Asthma | 2.4 | 1.05-5.2 | .036 |
| Chronic renal disease | 2.3 | 1.3-4.1 | .004 |
| Hypogammaglobulinemia | 0.67 | 0.39-1.1 | .14 |
| Smoking history (current vs never/former smoker) | 2.3 | 1.1-5.0 | .027 |
| Ever treated vs watch and wait | 0.88 | 0.53-1.4 | .60 |
| Currently treated vs observation | 0.70 | 0.42-1.1 | .15 |
| Current BTKi therapy | 0.80 | 0.47-1.4 | .42 |
| Prior lines of therapy (continuous variable) | 0.98 | 0.78-1.2 | .87 |
| Country of diagnosis | |||
| Spain vs United States | 1.2 | 0.71-2.1 | .47 |
| United Kingdom vs United States | 1.1 | 0.52-2.2 | .86 |
| Multivariable analyses | |||
| Age at COVID-19 diagnosis (≥75 y vs <75 y) | 1.8 | 1.1-3.0 | .028 |
| CIRS (>6 vs ≤6) | 1.6 | 1.0-2.9 | .043 |
| Diabetes | 1.5 | 0.8-2.5 | .172 |
| Asthma | 2.5 | 1.1-5.8 | .025 |
| Chronic renal disease | 1.8 | 1.0-3.4 | .035 |
| . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Univariable analyses | |||
| Sex (male vs female) | 0.66 | 0.40-1.1 | .10 |
| Age at COVID-19 diagnosis (≥75 vs <75 y) | 2.0 | 1.2-3.3 | .004 |
| CIRS (>6 vs ≤6) | 2.8 | 1.4-5.4 | .001 |
| Lymphopenia (ALC ≥1.0 × 109/L vs ALC <1.0 × 109/L) | 1.0 | 0.58-1.9 | .88 |
| Comorbidities | |||
| Hypertension | 1.4 | 0.88-2.4 | .137 |
| Diabetes | 2.0 | 1.2-3.3 | .011 |
| Arrhythmia | 1.5 | 0.87-2.7 | .13 |
| Coronary artery disease | 1.3 | 0.64-2.7 | .44 |
| COPD | 1.4 | 0.67-2.7 | .40 |
| Asthma | 2.4 | 1.05-5.2 | .036 |
| Chronic renal disease | 2.3 | 1.3-4.1 | .004 |
| Hypogammaglobulinemia | 0.67 | 0.39-1.1 | .14 |
| Smoking history (current vs never/former smoker) | 2.3 | 1.1-5.0 | .027 |
| Ever treated vs watch and wait | 0.88 | 0.53-1.4 | .60 |
| Currently treated vs observation | 0.70 | 0.42-1.1 | .15 |
| Current BTKi therapy | 0.80 | 0.47-1.4 | .42 |
| Prior lines of therapy (continuous variable) | 0.98 | 0.78-1.2 | .87 |
| Country of diagnosis | |||
| Spain vs United States | 1.2 | 0.71-2.1 | .47 |
| United Kingdom vs United States | 1.1 | 0.52-2.2 | .86 |
| Multivariable analyses | |||
| Age at COVID-19 diagnosis (≥75 y vs <75 y) | 1.8 | 1.1-3.0 | .028 |
| CIRS (>6 vs ≤6) | 1.6 | 1.0-2.9 | .043 |
| Diabetes | 1.5 | 0.8-2.5 | .172 |
| Asthma | 2.5 | 1.1-5.8 | .025 |
| Chronic renal disease | 1.8 | 1.0-3.4 | .035 |
OS from time of COVID-19 diagnosis stratified by age, CIRS score, treatment history, and use of BTKi at time of COVID-19 diagnosis. (A) OS stratified by age; (B) OS stratified by CIRS score; (C) OS by CLL treatment status; (D) OS by BTKi status. LR, log rank.
OS from time of COVID-19 diagnosis stratified by age, CIRS score, treatment history, and use of BTKi at time of COVID-19 diagnosis. (A) OS stratified by age; (B) OS stratified by CIRS score; (C) OS by CLL treatment status; (D) OS by BTKi status. LR, log rank.
With the caveat that most patients on BTKi’s at the time of symptomatic COVID-19 diagnosis had their BTKi held (79%), receiving a BTKi at the time of COVID-19 diagnosis did not appear to impact survival (case fatality rate: 30% for all patients receiving BTKi alone in or combination, 33% for patients receiving BTKi monotherapy, and 35% for patients not on BTKi’s). BTKi-treated patients diagnosed with symptomatic COVID-19 who stayed on therapy (n = 14) appeared to less frequently require supplemental oxygen (86%) and mechanical ventilation (21%). The subset of BTKi-treated patients who continued therapy had a case fatality rate of 21%.
COVID-19 management
The management strategies of COVID-19, including administration of antiviral therapies and anti-inflammatory agents aimed at the associated systemic inflammation syndrome, varied widely in this cohort (Table 4). COVID-19–directed therapies were administered as part of a clinical trial or compassionate use protocol in 16% and 19% of patients, respectively. Antiviral approaches included hydroxychloroquine (55%), lopinavir/ritonavir (17%), and remdesivir (7%). Eighty patients did not receive any of these antiviral agents. In addition, 48% received corticosteroids, 27% received azithromycin, 22% received tocilizumab, 7% received IV immunoglobulin, and 5% received convalescent plasma. Concomitant corticosteroids were administered to 65% of patients who received ≥1 antiviral agent. Concomitant tocilizumab was administered to 32% of patients who received ≥1 antiviral agent. For patients requiring mechanical ventilation, 63% received hydroxychloroquine, 65% received corticosteroids, and 40% received tocilizumab. For patients receiving CLL-directed therapy at the time of COVID-19 diagnosis, the majority (88%) underwent treatment interruption at or near the time of COVID-19 diagnosis.
For 139 (70%) patients receiving COVID-19 based therapy, defined as ≥1 antiviral (hydroxychloroquine, lopinavir/ritonavir, remdesivir, or convalescent plasma) or 1 anti-inflammatory agent (corticosteroids or tocilizumab), the case fatality rate was 36%. Baseline characteristics were similar in admitted patients stratified by hydroxychloroquine exposure (supplemental Table 1), and OS was similar between these groups (supplemental Figure 2).
Discussion
As the COVID-19 pandemic has caused severe morbidity and high mortality in patients affected across the globe, outcomes data for cancer patients developing COVID-19 have been heterogeneous. The data that exist broadly suggest that patients with hematologic malignancies are particularly susceptible to SARS-CoV2 infection and vulnerable to severe manifestations of COVID-19.27,28
To the best of our knowledge, we describe the first large, disease-specific series in a defined cohort of hematologic cancer patients. Given that CLL patients have impaired humoral and cellular immune function, we hypothesized that this cohort might be at particular risk of severe COVID-19 with its associated morbidity, including superimposed infections,20 and mortality. We aimed to better define this risk and determine the relationship between patient or disease characteristics and outcomes for patients with CLL and symptomatic COVID-19. Herein, we report several key findings with direct clinical relevance.
First, at a median follow-up of 16 days, CLL patients with symptomatic COVID-19 have a high mortality rate when requiring inpatient admission (37%). Further, 49 patients who were admitted remain as inpatients at the time of analysis, suggesting that case fatality rate for inpatients in this series will rise beyond 37%. For the 129 patients who have been discharged or have died, the case fatality rate is 50%. These mortality rates appear to be at least similar, if not unfavorable, compared with large series of all-comer symptomatic COVID-19 patients requiring hospital admission. Series of admitted patients in New York City show mortality rates ranging from 10% to 22%.4-7 A UK series of 16 749 patients described a death rate of 33%, with 17% continuing to receive inpatient care at the time of reporting.8 Data across all series presented to date are relatively immature, and metrics are presented variably. These factors, in addition to standard caveats associated with cross-study comparisons, mandate significant caution in making comparison. Despite this, our data suggest that CLL patients suffer from a high case fatality rate when requiring hospital admission with symptomatic COVID-19.
Second, we found no differences in OS for patients who have received CLL-directed therapy vs the watch-and-wait population. This remained true even after adjusting for key parameters, including age, CIRS score, diabetes, chronic renal disease, and smoking status. It has been hypothesized that the humoral and cellular immune dysfunction of untreated CLL may put watch-and-wait patients at particular risk. Patients who have not received therapy did not suffer a more aggressive course in our cohort, as they experienced similar rates of admission, ICU admission, and need for intubation as patients who had received CLL-directed therapy. Initial efforts have suggested low prevalence (<1%) in CLL populations,20 though the risk of acquiring infection and the rate of asymptomatic or mild symptomatic COVID-19 remain unclear among CLL patients in the absence of widespread community testing. As such, it remains challenging to draw strong conclusions about the absolute or relative risk of SARS-CoV-2 infection in these 2 patient cohorts. Additionally, patients with CLL are known to produce suboptimal response to available vaccines, and a recent small recent series has suggested that cancer patients may not mount full antibody responses to SARS-CoV-2; however, we cannot conclude whether CLL patients may be at higher risk for failure to mount an immune response to potential SARS-CoV-2 vaccines.29
Third, BTKi use was the most common active CLL treatment in our cohort with symptomatic COVID-19 and did not seem to impact OS. It has been postulated that inhibition of BTK could modulate immune response through blockade of proinflammatory and chemoattractant cytokines in lung tissue and a shift from an M1 to M2 polarized macrophage, thereby mitigating hyperinflammatory response.22-24 In our series, the majority of patients on BTKi had their drug held during their COVID-19 course, while a small number of patients had a numerical but nonsignificant improvement in case fatality rate. Whether this observation suggests a protective effect of BTKi’s or is merely a consequence of less severe disease in the population for whom BTKi was continued, thus not prompting a change in CLL-directed therapy, remains unknown. Randomized clinical trials of BTKi’s for the treatment of COVID-19 are ongoing and will provide more definitive evidence of the effect of these drugs in COVID-19 (NCT04375397 and NCT04380688).
Fourth, we demonstrate that within a CLL-specific inpatient population, the additional risk factors of age ≥75 years, CIRS score >6, underlying chronic renal disease, and asthma were independent predictors of poor survival. These data show that known risk factors from non–cancer population–based data also modulate outcomes in CLL patients. Recent data from the UK Coronavirus Cancer Monitoring Project also found that age, male gender, and comorbidity burden are associated with worse outcome within a heterogenous population of 800 cancer patients.9 Robust analysis of risk factors within cancer-specific populations are critically important to identify patients at particularly high risk of adverse outcomes, or conversely, to define lower risk populations for whom extremely strict social distancing measures may not be as essential.
While we did not aim to specifically examine efficacy of treatment options for CLL patients with symptomatic COVID-19, we did not find OS differences in admitted patients who received hydroxychloroquine therapy as compared with those who did not receive hydroxychloroquine. These findings are consistent with the key findings of the randomized RECOVERY trial, where no reduction in mortality was shown in 1542 patients randomized to hydroxychloroquine or 3132 patients to supportive care (end point of 28-day mortality: 25.7% for hydroxychloroquine vs 23.5% for supportive care; HR, 1.11; 95% CI, 0.98-1.26; P = .10). There was also no evidence of beneficial effects on hospital stay duration outcomes.30
We recognize several limitations of our study. It is retrospective and hypothesis-generating only. The follow-up of this patient population is short and will require longer follow-up to more fully understand the impact of COVID-19 on CLL patients. We fully recognize the possibility for ascertainment bias in this cohort and emphasize that our cohort represents CLL patients with symptomatic COVID-19. We acknowledge that we have not captured asymptomatic carriers, patients who were mildly symptomatic and did not undergo testing, or those who died with unrecognized COVID-19. Inclusion of these patients may significantly affect case fatality rates. In addition, we recognize that testing availability, criteria for testing, and accuracy of SARS-CoV-2 tests are not uniform worldwide. We also acknowledge that our study aims did not assess COVID-19 incidence and prevalence in our respective practices; however, we note that no such data sets are available for any specific malignancy. Practically speaking, while we would have liked to perform polymerase chain reaction–based testing/serologic testing on a representative sample of all CLL patients with CLL to better understand COVID-19 epidemiology, this was not possible at the height of the pandemic due to limited testing resources. In addition, the medical community advised asymptomatic CLL patients to shelter in place. These factors may have impacted the cohort of patients captured in this study. Regarding COVID-19 management, practices surrounding disease management were heterogeneous in the setting of an evolving pandemic, with no established standard of care. Thus, this study has intentionally not commented on the optimal management strategy of COVID-19 or analyzed the data in that regard apart from confirming prior reports that hydroxychloroquine did not appear to improve OS. We also acknowledge that we broadly have studied a young CLL population with a relatively high proportion of patients on treatment at the time of symptomatic COVID-19 diagnosis. As in other real-world series, this most likely reflects the nature of large specialist centers involved in this study. Additionally, while this study suggests that CLL treatment status and receiving BTKi’s does not impact outcome within this inpatient cohort, practices surrounding management of CLL-directed therapy at the time of COVID-19 diagnosis are heterogeneous, thus limiting the ability to draw definitive conclusions. Larger data sets are needed to validate our initial findings. Further, widespread community testing, including patients with CLL, is needed to give a complete picture of the burden of COVID-19 in CLL patients, the true case fatality rate, and a better understanding of potential protective factors in the early manifestations of the disease. Finally, at the time of study initiation, there were many aspects of the clinical presentation of COVID-19 that were not fully described in the literature and were not captured.
Despite these limitations, we provide outcomes on the largest cohort of patients with hematological cancer in the literature to date. Among the subgroup of CLL patients requiring hospital inpatient admission for symptomatic COVID-19, we found a similar if not numerically higher mortality rate compared with other population-based data sets studying similar inpatient metrics. Known risk factors for mortality in COVID-19, including age, increasing comorbidity burden, asthma, and chronic renal disease, remain significant in this population of CLL patients. Watch-and-wait patients and those who have received CLL treatment have similar outcomes, independent of disease phase, and we found no clear protective or harmful effect for patients on BTKi’s at the time of symptomatic COVID-19 diagnosis.
Data may be made available by e-mail request for consideration to the study chairs (A.R.M. and L.E.R.) and establishment of data transfer agreement.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank Katherine Whittaker and Elizabeth Chamberlain for administrative support and Lee Stetson for legal support in negotiating data transfer agreements.
This research was supported in part by the National Institutes of Health/National Cancer Institute (Cancer Center Support Grant P30 CA008748). L.E.R. recognizes support from the American Society of Hematology Research Training Award for Fellows outside of the submitted work. I.E.A. receives research support from the Intramural Program of the National Heart, Lung, and Blood Institute and an American Society of Hematology Scholar Award. T.A.E. recognizes support from the Oxford NIHR Biomedical Research Centre.
Authorship
Contribution: A.R.M. and L.E.R. are the study principal investigators (PIs) and contributed to study design, data collection, coordination, analysis, and interpretation, and manuscript writing; N.L., J.N.A., L.L., J.M.P., K.P., A.O., D.W., M. Kamdar, S.F.H., M.S.D., J.R.B., D.A., R.J., I.E.A., J.P., K.M.I., P.M.B., C.S.U., M.B.G., E. Berman, A.D.Z., N.M., R.R.F., M. Koropsak, N.B., L.H., G.F.P., S.M., C.E.R., A.W., C.A.P., M.S., E.A.C., S.S., A.N.S., E.S., M.P., N.M.-C., T.M., R.W., A.B., H.W., D.E.-S., H.P., M.R.W., P.E.M.P., J.-Á.H.-R., F.M., N.F.E., P.G., S.L., E. Bhavsar, J.L.-J., D.N., J.A.G.-M., and S.S.S. contributed to data collection and interpretation and manuscript editing; D.M.B. and C.N. contributed to study design, data interpretation, and manuscript writing and editing; and R.C. and T.A.E. contributed to study design, site coordination, data collection and interpretation, and manuscript writing and editing.
Conflict-of-interest disclosure: A.R.M. received grants, personal fees, DSMB membership, and other funds from TG Therapeutics; grants and personal fees from Pharmacyclics, Janssen, Genentch, AbbVie, Adaptive, and Astra Zeneca; grants and other funds from Celgene; grants from Loxo, Sunesis, Regeneron, and DTRM Biopharm; and personal fees from BeiGene. L.E.R. received grant support from the American Society of Hematology and has minority ownership interest in AbbVie and Abbott Laboratories. J.N.A. received advisory/consulting fees from AbbVie, Pharmacyclics, Janssen, AstraZeneca, and Genentech and honoraria and nonpromotional speaking fees from Janssen, AbbVie, and Pharmacyclics. L.L. received speakers bureau fees from Seattle Genetics, Celgene/BMS, KitePharma, BeiGene, Pharmacyclics/Janssen, and AstraZeneca and advisory board participation fees from Bayer, Seattle Genetics, ADC Therapeutics, AbbVie, Janssen, Pharmacyclics, KitePharma, and AstraZeneca. A.O. received grants for academic research from BeiGene and Kancera, has stock ownership in Kancera, and received consultancy fees from Sanofi. S.F.H. is a consultant for Celgene, Bayer, Genentech, Pharmacyclics, Novartis, and AbbVie and received research funding from DTRM Biopharm, Celgene, and TG Therapeutics. M.S.D. received grants from Ascentage Pharma, AstraZeneca, BMS, Genentech, MEI Pharma, Pharmacyclics, Surface Oncology, TG Therapeutics and Verastem, and consulting fees from AbbVie, Adaptive Biotechnologies, Ascentage Pharma, AstraZeneca, BeiGene, Celgene, Genentech, Gilead Sciences, Janssen, MEI Pharma, Pharmacyclics, Syros Pharmaceuticals, TG Therapeutics, Verastem, and Zentalis. J.R.B. has served as a consultant for AbbVie, Acerta, AstraZeneca, BeiGene, Catapult, Dynamo Therapeutics, Juno/Celgene, KitePharma, MEI Pharma, Nextcea, Novartis, Octapharma, Pfizer, Sunesis, TG Therapeutics, and Verastem; received honoraria from Janssen and Teva; received research funding from Gilead, Loxo, Sun, and Verastem; and served on data safety monitoring committees for Morphosys and Invectys. P.M.B. received consulting fees for PCYC/AbbVie, Genentech, Gilead, Merck, Seattle Genetics, Verastem, AstraZeneca, Celgene, and Morphosys. C.S.U. received consulting fees from Pharmacyclics, AbbVie, and AstraZeneca. M.B.G. received research support from Amgen. M. Kamdar is a consultant for AZD, Celgene, and Pharmacyclics and received speakers bureau fees from Seattle Genetics. L.H. received research grant support from Gilead and Janssen-Cilag and honoraria from AbbVie; S.M. received research funding from AbbVie, AstraZeneca, BeiGene, Genentech, Gilead, Janssen, Juno, Novartis, Pharmacyclics, and TG Therapeutics and received honorarium for advisory board or lecturing for AbbVie, AstraZeneca, BeiGene, Genentech, Gilead, Janssen, KitePharma, and Pharmacyclics. M.S. received research funding from Mustang Bio, Celgene, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, Acerta Pharma, Merck, and has served on advisory boards or as a consultant for AbbVie, Genentech, Astra Zeneca, Sound Biologics, Verastem, ADC Therapeutics, BMS, and Atara Biotherapeutics. E.A.C. is on advisory boards for Novartis, Tessa, BMS, and KitePharma and received research funding from the Lymphoma Research Foundation. D.M.B. is a consultant, scientific advisory board member, and site PI clinical trial (grant paid to institution) for AbbVie, Juno/Celgene/BMS, and Tolero; a scientific advisory board member and site PI clinical trial (grant paid to institution) for ArQule; a site PI clinical trial (grant paid to institution) for Ascentage, BeiGene, DTRM Biopharm, Genentech, MEI Pharma, Pharmacyclics, and Verastem; a consultant and site PI clinical trial (grant paid to institution) for AstraZeneca; a consultant and scientific advisory board member for Pfizer; a consultant for Teva; a scientific advisory board member and site PI clinical trial (grant paid to institution) for TG Therapeutics; and has other guidelines/registry memberships (when sponsored or consultant also included under sponsor above) as a National Comprehensive Cancer Network panel member, informCLL registry steering committee (AbbVie), REAL registry steering committee (Verastem), and Biosimilars outcomes research panel (Pfizer). R.W. attends meetings and gives educational talks for AbbVie, Janssen, and Gilead. A.B. received travel expenses and speakers fees from Gilead; received travel/conference support from PEMP AbbVie; is a remunerated speaker and consultant for Astra Zeneca; is a remunerated speaker and consultant for Atura; is a consultant for Gilead; received research funding and travel/conference support and is a remunerated speaker and consultant for Janssen; received travel/conference support and is a remunerated speaker and consultant for Novartis, Roche, and Tolero Pharmaceuticals. J.-Á.H.-R. is a consultant for Janssen, AbbVie, Roche, Gilead, Celgene, AstraZeneca, and Rovi; received speakers bureau fees from Janssen, AbbVie, Roche, Gilead, Celgene, and AstraZeneca; and received grant/research support from Celgene and is a major shareholder. R.C. received speakers bureau fees from Roche, Janssen, BMS, AbbVie, and Takeda; received advisory fees from Janssen, Celgene, AbbVie, Servier, Kyowa-Kirin, and Takeda; and received travel and accommodation fees from Roche, Pfizer, Janssen, Celgene, AbbVie, Servier, and Takeda. T.A.E. received honoraria from Roche, Gilead, AbbVie, and Janssen; holds an advisory board role for Gilead, AbbVie, and AstraZeneca; has received research funding from Gilead; and has travelled to conferences for Takeda, AbbVie, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Anthony R. Mato, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: matoa@mskcc.org.
REFERENCES
Author notes
A.R.M. and L.E.R. contributed equally to this study.
R.C. and T.A.E. contributed equally to this study.