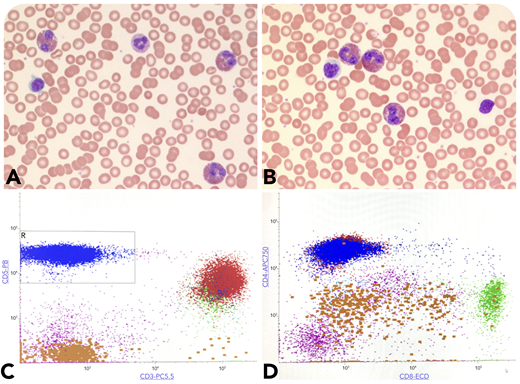
A 27-year-old woman presented with eczema-like lesions. Complete blood counts showed leukocytosis (13 500/μL) and absolute eosinophilia (5260/μL) (panels A-B; original magnification ×100 and ×400, respectively; Wright-Giemsa stain) with 19% lymphocytes. There were no circulating atypical lymphoid cells, blasts, or granulocytic dysplasia in the peripheral smear. Serum tryptase levels were normal, and no lymphadenopathy was found. Flow cytometry of the blood demonstrated an abnormal T-cell population (48% of lymphocytes), with loss of surface CD3 and CD7 expressing CD5, CD4, and CD2 and negative for CD8 (panels C-D, blue population). Genetic studies including fluorescence in situ hybridization and next-generation sequencing were negative for abnormalities in PDGFRA, PDGFRB, FGFR1, JAK2, and BCR/ABL1, excluding myeloid neoplasm-related eosinophilia. An underlying clonal T-cell receptor gene rearrangement was detected and supported the diagnosis of lymphocytic variant of hypereosinophilic syndrome (L-HES). An additional bone marrow biopsy showed <4% blasts with increased eosinophils without abnormal lymphoid infiltrates.
L-HES should be considered in the differentials for hypereosinophilia (Sezary syndrome, adult T-cell leukemia, and myeloid neoplasms). Similar abnormal CD3−/CD4+ T-cell populations have been described in angioimmunoblastic T-cell lymphoma. A correct diagnosis has significant therapeutic implications, notably because of dependency on corticosteroids and steroid-sparing agents in L-HES, as opposed to aggressive chemotherapy. Increased interleukin-4 (IL-4), IL-5, and IL-13 production by abnormal clonal CD3−/CD4+ T cells is thought to underlie the eosinophilia associated with elevated serum TARC levels, a Th2 cytokine in L-HES.
A 27-year-old woman presented with eczema-like lesions. Complete blood counts showed leukocytosis (13 500/μL) and absolute eosinophilia (5260/μL) (panels A-B; original magnification ×100 and ×400, respectively; Wright-Giemsa stain) with 19% lymphocytes. There were no circulating atypical lymphoid cells, blasts, or granulocytic dysplasia in the peripheral smear. Serum tryptase levels were normal, and no lymphadenopathy was found. Flow cytometry of the blood demonstrated an abnormal T-cell population (48% of lymphocytes), with loss of surface CD3 and CD7 expressing CD5, CD4, and CD2 and negative for CD8 (panels C-D, blue population). Genetic studies including fluorescence in situ hybridization and next-generation sequencing were negative for abnormalities in PDGFRA, PDGFRB, FGFR1, JAK2, and BCR/ABL1, excluding myeloid neoplasm-related eosinophilia. An underlying clonal T-cell receptor gene rearrangement was detected and supported the diagnosis of lymphocytic variant of hypereosinophilic syndrome (L-HES). An additional bone marrow biopsy showed <4% blasts with increased eosinophils without abnormal lymphoid infiltrates.
L-HES should be considered in the differentials for hypereosinophilia (Sezary syndrome, adult T-cell leukemia, and myeloid neoplasms). Similar abnormal CD3−/CD4+ T-cell populations have been described in angioimmunoblastic T-cell lymphoma. A correct diagnosis has significant therapeutic implications, notably because of dependency on corticosteroids and steroid-sparing agents in L-HES, as opposed to aggressive chemotherapy. Increased interleukin-4 (IL-4), IL-5, and IL-13 production by abnormal clonal CD3−/CD4+ T cells is thought to underlie the eosinophilia associated with elevated serum TARC levels, a Th2 cytokine in L-HES.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal